Monomethylarsonous acid produces irreversible events resulting in malignant transformation of a human bladder cell line following 12 weeks of low-level exposure
- PMID: 20375083
- PMCID: PMC2886861
- DOI: 10.1093/toxsci/kfq106
Monomethylarsonous acid produces irreversible events resulting in malignant transformation of a human bladder cell line following 12 weeks of low-level exposure
Abstract
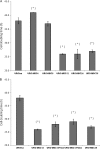
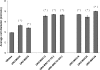
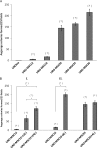
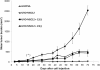
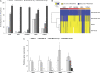
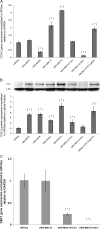
Arsenic is a known human bladder carcinogen; however, the mechanisms underlying arsenical-induced bladder carcinogenesis are not understood. Previous research has demonstrated that exposure of a nontumorigenic human urothelial cell line, UROtsa, to 50 nM monomethylarsonous acid (MMA(III)) for 52 weeks resulted in malignant transformation. To focus research on the early mechanistic events leading to MMA(III)-induced malignancy, the goal of this research was to resolve the critical period in which continuous MMA(III) exposure (50 nM) induces the irreversible malignant transformation of UROtsa cells. An increased growth rate of UROtsa cells results after 12 weeks of MMA(III) exposure. Anchorage-independent growth occurred after 12 weeks with a continued increase in colony formation when 12-week exposed cells were cultured for an additional 12 or 24 weeks without MMA(III) exposure. UROtsa cells as early as 12 weeks MMA(III) exposure were tumorigenic in severe combined immunodeficiency mice with tumorigenicity increasing when 12-week exposed cells were cultured for an additional 12 or 24 weeks in the absence of MMA(III) exposure. To assess potential underlying mechanisms associated with the early changes that occur during MMA(III)-induced malignancy, DNA methylation was assessed in known target gene promoter regions. Although DNA methylation remains relatively unchanged after 12 weeks of exposure, aberrant DNA methylation begins to emerge after an additional 12 weeks in culture and continues to increase through 24 weeks in culture without MMA(III) exposure, coincident with the progression of a tumorigenic phenotype. Overall, these data demonstrate that 50 nM MMA(III) is capable of causing irreversible malignant transformation in UROtsa cells after 12 weeks of exposure. Having resolved an earlier timeline in which MMA(III)-induced malignant transformation occurs in UROtsa cells will allow for mechanistic studies focused on the critical biological changes taking place within these cells prior to 12 weeks of exposure, providing further evidence about potential mechanisms of MMA(III)-induced carcinogenesis.
Figures
References
-
- Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J. Natl. Cancer Inst. 2002;94:1888–1891. - PubMed
-
- Aposhian HV, Gurzau ES, Le XC, Gurzau A, Healy SM, Lu X, Ma M, Yip Li, Zakharyan RA, Maiorina RM, et al. Occurrence of monomethylarsonous acid in urine of humans exposed to inorganic arsenic. Chem. Res. Toxicol. 2000;13:693–697. - PubMed
-
- Benbrahim-Tallaa L, Waterland RA, Styblo M, Achanzar WE, Webber MM, Waalkes MP. Molecular events associated with arsenic-induced malignant transformation of human prostatic epithelial cells: aberrant genomic DNA methylation and K-ras oncogene activation. Toxicol. Appl. Pharmacol. 2005;206:288–298. - PubMed
-
- Bredfeldt TG, Kopplin MJ, Gandolfi AJ. Effects of arsenite on UROtsa cells: low-level arsenite causes accumulation of ubiquitinated proteins that is enhanced by reduction in cellular glutathione levels. Toxicol. Appl. Pharmacol. 2004;198:412–418. - PubMed

