Spinal cord injury causes a wide-spread, persistent loss of Kir4.1 and glutamate transporter 1: benefit of 17 beta-oestradiol treatment
- PMID: 20375134
- PMCID: PMC2850584
- DOI: 10.1093/brain/awq049
Spinal cord injury causes a wide-spread, persistent loss of Kir4.1 and glutamate transporter 1: benefit of 17 beta-oestradiol treatment
Abstract
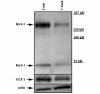
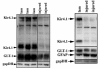
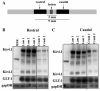
During neuronal activity astrocytes function to remove extracellular increases in potassium, which are largely mediated by the inwardly-rectifying potassium channel Kir4.1, and to take up excess glutamate via glutamate transporter 1, a glial-specific glutamate transporter. Here we demonstrate that expression of both of these proteins is reduced by nearly 80% following a crush spinal cord injury in adult male rats, 7 days post-injury. This loss extended to spinal segments several millimetres rostral and caudal to the lesion epicentre, and persisted at 4 weeks post-injury. Importantly, we demonstrate that loss of these two proteins is not a direct result of astrocyte loss, as immunohistochemistry at 7 days and western blots at 4 weeks demonstrate a marked up-regulation in glial fibrillary acidic protein expression. Kir4.1 and glutamate transporter 1 expression were partially rescued by post-spinal cord injury administration of physiological levels of 17beta-oestradiol (0.08 mg/kg/day) in vivo. Utilizing an in vitro culture system we demonstrate that 17beta-oestradiol treatment (50 nM) is sufficient to increase glutamate transporter 1 protein expression in spinal cord astrocytes. This increase in glutamate transporter 1 protein expression was reversed and Kir4.1 expression reduced in the presence of an oestrogen receptor antagonist, Fulvestrant 182,780 suggesting a direct translational regulation of Kir4.1 and glutamate transporter 1 via genomic oestrogen receptors. Using whole-cell patch-clamp recordings in cultured spinal cord astrocytes, we show that changes in protein expression following oestrogen application led to functional changes in Kir4.1 mediated currents. These findings suggest that the neuroprotective benefits previously seen with 17beta-oestradiol after spinal cord injury may be in part due to increased Kir4.1 and glutamate transporter 1 expression in astrocytes leading to improved potassium and glutamate homeostasis.
Figures




Similar articles
-
Differential distribution of Kir4.1 in spinal cord astrocytes suggests regional differences in K+ homeostasis.J Neurophysiol. 2007 Aug;98(2):786-93. doi: 10.1152/jn.00340.2007. Epub 2007 Jun 20. J Neurophysiol. 2007. PMID: 17581847 Free PMC article.
-
Overexpression of the astrocyte glutamate transporter GLT1 exacerbates phrenic motor neuron degeneration, diaphragm compromise, and forelimb motor dysfunction following cervical contusion spinal cord injury.J Neurosci. 2014 May 28;34(22):7622-38. doi: 10.1523/JNEUROSCI.4690-13.2014. J Neurosci. 2014. PMID: 24872566 Free PMC article.
-
Functional Indicators of Glutamate Transport in Single Striatal Astrocytes and the Influence of Kir4.1 in Normal and Huntington Mice.J Neurosci. 2016 May 4;36(18):4959-75. doi: 10.1523/JNEUROSCI.0316-16.2016. J Neurosci. 2016. PMID: 27147650 Free PMC article.
-
Emerging Roles of Astrocyte Kir4.1 Channels in the Pathogenesis and Treatment of Brain Diseases.Int J Mol Sci. 2021 Sep 23;22(19):10236. doi: 10.3390/ijms221910236. Int J Mol Sci. 2021. PMID: 34638578 Free PMC article. Review.
-
Inwardly Rectifying Potassium Channel Kir4.1 as a Novel Modulator of BDNF Expression in Astrocytes.Int J Mol Sci. 2018 Oct 24;19(11):3313. doi: 10.3390/ijms19113313. Int J Mol Sci. 2018. PMID: 30356026 Free PMC article. Review.
Cited by
-
Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-L-lactic acid fibers.Biomaterials. 2014 Feb;35(5):1439-49. doi: 10.1016/j.biomaterials.2013.10.079. Epub 2013 Nov 15. Biomaterials. 2014. PMID: 24246642 Free PMC article.
-
In vivo microdialysis of glutamate in ventroposterolateral nucleus of thalamus following electrolytic lesion of spinothalamic tract in rats.Exp Brain Res. 2014 Feb;232(2):415-21. doi: 10.1007/s00221-013-3749-0. Epub 2013 Nov 2. Exp Brain Res. 2014. PMID: 24186197
-
Linolenic acid provides multi-cellular protective effects after photothrombotic cerebral ischemia in rats.Neurochem Res. 2014 Sep;39(9):1797-808. doi: 10.1007/s11064-014-1390-3. Epub 2014 Jul 26. Neurochem Res. 2014. PMID: 25062759
-
Rapid Generation of Ventral Spinal Cord-like Astrocytes from Human iPSCs for Modeling Non-Cell Autonomous Mechanisms of Lower Motor Neuron Disease.Cells. 2022 Jan 24;11(3):399. doi: 10.3390/cells11030399. Cells. 2022. PMID: 35159209 Free PMC article.
-
A toll-like receptor 9 antagonist restores below-level glial glutamate transporter expression in the dorsal horn following spinal cord injury.Sci Rep. 2018 Jun 7;8(1):8723. doi: 10.1038/s41598-018-26915-2. Sci Rep. 2018. PMID: 29880832 Free PMC article.
References
-
- Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. - PubMed
-
- Chaovipoch P, Jelks KA, Gerhold LM, West EJ, Chongthammakun S, Floyd CL. 17beta-estradiol is protective in SC injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–52. - PubMed
-
- Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol. 2007;42:70–5. - PubMed
-
- Dibaj P, Kaiser M, Hirrlinger J, Kirchhoff F, Neusch C. Kir4.1 channels regulate swelling of astroglial processes in experimental SC edema. J Neurochem. 2007;103:2620–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

