Axon initial segment dysfunction in epilepsy
- PMID: 20375142
- PMCID: PMC2901971
- DOI: 10.1113/jphysiol.2010.188417
Axon initial segment dysfunction in epilepsy
Abstract
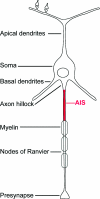
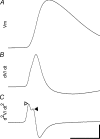
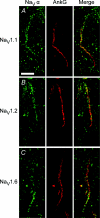
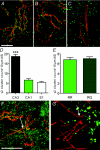
The axon initial segment (AIS) contains the site of action potential initiation and plays a major role in neuronal excitability. AIS function relies on high concentrations of different ion channels and complex regulatory mechanisms that orchestrate molecular microarchitecture. We review recent evidence that a large number of ion channels associated with epilepsy are enriched at the AIS, making it a 'hotspot' for epileptogenesis. Furthermore, we present novel data on the clustering of GABRgamma2 receptors in the AIS of cortical and hippocampal neurons in a knock in mouse model of a human genetic epilepsy. This article highlights the molecular coincidence of epilepsy mutations at the AIS and reviews pathogenic mechanisms converging at the AIS.
Figures
References
-
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Bioph Intern. 2004;11:36–42.
-
- Araki T, Otani T. Response of single motoneurons to direct stimulation in toad's spinal cord. J Neurophysiol. 1955;18:472–485. - PubMed
-
- Audenaert D, Claes L, Ceulemans B, Lofgren A, Van Broeckhoven C, De Jonghe P. A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology. 2003;61:854–856. - PubMed
-
- Audenaert D, Van Broeckhoven C, De Jonghe P. Genes and loci involved in febrile seizures and related epilepsy syndromes. Hum Mutat. 2006;27:391–401. - PubMed
-
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nat Genet. 2001;28:46–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

