A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3L for glioma therapeutics
- PMID: 20375153
- PMCID: PMC2876634
- DOI: 10.1128/JVI.00398-10
A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3L for glioma therapeutics
Abstract
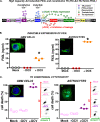
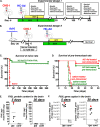
Glioblastoma multiforme (GBM) is a deadly primary brain tumor. Conditional cytotoxic/immune-stimulatory gene therapy (Ad-TK and Ad-Flt3L) elicits tumor regression and immunological memory in rodent GBM models. Since the majority of patients enrolled in clinical trials would exhibit adenovirus immunity, which could curtail transgene expression and therapeutic efficacy, we used high-capacity adenovirus vectors (HC-Ads) as a gene delivery platform. Herein, we describe for the first time a novel bicistronic HC-Ad driving constitutive expression of herpes simplex virus type 1 thymidine kinase (HSV1-TK) and inducible Tet-mediated expression of Flt3L within a single-vector platform. We achieved anti-GBM therapeutic efficacy with no overt toxicities using this bicistronic HC-Ad even in the presence of systemic Ad immunity. The bicistronic HC-Ad-TK/TetOn-Flt3L was delivered into intracranial gliomas in rats. Survival, vector biodistribution, neuropathology, systemic toxicity, and neurobehavioral deficits were assessed for up to 1 year posttreatment. Therapeutic efficacy was also assessed in animals preimmunized against Ads. We demonstrate therapeutic efficacy, with vector genomes being restricted to the brain injection site and an absence of overt toxicities. Importantly, antiadenoviral immunity did not inhibit therapeutic efficacy. These data represent the first report of a bicistronic vector platform driving the expression of two therapeutic transgenes, i.e., constitutive HSV1-TK and inducible Flt3L genes. Further, our data demonstrate no promoter interference and optimum gene delivery and expression from within this single-vector platform. Analysis of the efficacy, safety, and toxicity of this bicistronic HC-Ad vector in an animal model of GBM strongly supports further preclinical testing and downstream process development of HC-Ad-TK/TetOn-Flt3L for a future phase I clinical trial for GBM.
Figures



Similar articles
-
Safety profile, efficacy, and biodistribution of a bicistronic high-capacity adenovirus vector encoding a combined immunostimulation and cytotoxic gene therapy as a prelude to a phase I clinical trial for glioblastoma.Toxicol Appl Pharmacol. 2013 May 1;268(3):318-30. doi: 10.1016/j.taap.2013.02.001. Epub 2013 Feb 9. Toxicol Appl Pharmacol. 2013. PMID: 23403069 Free PMC article.
-
Safety profile of gutless adenovirus vectors delivered into the normal brain parenchyma: implications for a glioma phase 1 clinical trial.Hum Gene Ther Methods. 2012 Aug;23(4):271-84. doi: 10.1089/hgtb.2012.060. Epub 2012 Sep 5. Hum Gene Ther Methods. 2012. PMID: 22950971 Free PMC article.
-
Optimization of adenoviral vector-mediated transgene expression in the canine brain in vivo, and in canine glioma cells in vitro.Neuro Oncol. 2007 Jul;9(3):245-58. doi: 10.1215/15228517-2007-012. Epub 2007 May 23. Neuro Oncol. 2007. PMID: 17522335 Free PMC article.
-
Combined cytotoxic and immune-stimulatory gene therapy using Ad-TK and Ad-Flt3L: Translational developments from rodents to glioma patients.Mol Ther. 2023 Oct 4;31(10):2839-2860. doi: 10.1016/j.ymthe.2023.08.009. Epub 2023 Aug 12. Mol Ther. 2023. PMID: 37574780 Free PMC article. Review.
-
Adenoviral vector-mediated gene therapy for gliomas: coming of age.Expert Opin Biol Ther. 2014 Sep;14(9):1241-57. doi: 10.1517/14712598.2014.915307. Epub 2014 Apr 29. Expert Opin Biol Ther. 2014. PMID: 24773178 Free PMC article. Review.
Cited by
-
Human Organotypic Cultured Cardiac Slices: New Platform For High Throughput Preclinical Human Trials.Sci Rep. 2016 Jun 30;6:28798. doi: 10.1038/srep28798. Sci Rep. 2016. PMID: 27356882 Free PMC article.
-
Current status of gene therapy for brain tumors.Transl Res. 2013 Apr;161(4):339-54. doi: 10.1016/j.trsl.2012.11.003. Epub 2012 Dec 11. Transl Res. 2013. PMID: 23246627 Free PMC article.
-
Overview of current immunotherapeutic strategies for glioma.Immunotherapy. 2015;7(10):1073-104. doi: 10.2217/imt.15.75. Immunotherapy. 2015. PMID: 26598957 Free PMC article. Review.
-
Preclinical Evaluation of a Replication-Deficient Recombinant Adenovirus Serotype 5 Vaccine Expressing Guanylate Cyclase C and the PADRE T-helper Epitope.Hum Gene Ther Methods. 2016 Dec;27(6):238-250. doi: 10.1089/hgtb.2016.114. Hum Gene Ther Methods. 2016. PMID: 27903079 Free PMC article.
-
Development of preclinical models for immunogene therapy of brain cancer: it's not monkey business!Mol Ther. 2014 Feb;22(2):247-249. doi: 10.1038/mt.2013.298. Mol Ther. 2014. PMID: 24487565 Free PMC article. No abstract available.
References
-
- Ali, S., J. F. Curtin, J. M. Zirger, W. Xiong, G. D. King, C. Barcia, C. Liu, M. Puntel, S. Goverdhana, P. R. Lowenstein, and M. G. Castro. 2004. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol. Ther. 10:1071-1084. - PMC - PubMed
-
- Ali, S., G. D. King, J. F. Curtin, M. Candolfi, W. Xiong, C. Liu, M. Puntel, Q. Cheng, J. Prieto, A. Ribas, J. Kupiec-Weglinski, N. van Rooijen, H. Lassmann, P. R. Lowenstein, and M. G. Castro. 2005. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 65:7194-7204. - PMC - PubMed
-
- Ark Therapeutics Group. 7 April 2009, posting date. Cerepro phase III trial update. http://www.drugs.com/clinical_trials/cerepro-phase-iii-trial-update-6970....
-
- Barcia, C., M. Jimenez-Dalmaroni, K. M. Kroeger, M. Puntel, A. J. Rapaport, D. Larocque, G. D. King, S. A. Johnson, C. Liu, W. Xiong, M. Candolfi, S. Mondkar, P. Ng, D. Palmer, M. G. Castro, and P. R. Lowenstein. 2007. Sustained, one year expression from high-capacity helper-dependent adenoviral vectors delivered to the brain of animals with a pre-existing systemic anti-adenoviral immune response: implications for clinical trials. Mol. Ther. 15:2154-2163. - PMC - PubMed
-
- Candolfi, M., J. F. Curtin, W. S. Nichols, A. G. Muhammad, G. D. King, G. E. Pluhar, E. A. McNiel, J. R. Ohlfest, A. B. Freese, P. F. Moore, J. Lerner, P. R. Lowenstein, and M. G. Castro. 2007. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J. Neurooncol. 85:133-148. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

