Through its nonstructural protein NS1, parvovirus H-1 induces apoptosis via accumulation of reactive oxygen species
- PMID: 20375165
- PMCID: PMC2876649
- DOI: 10.1128/JVI.01797-09
Through its nonstructural protein NS1, parvovirus H-1 induces apoptosis via accumulation of reactive oxygen species
Abstract
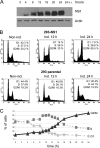
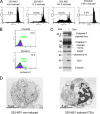
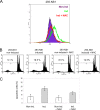
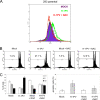
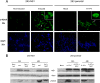
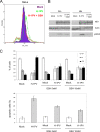
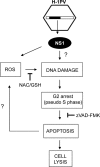
The rat parvovirus H-1 (H-1PV) attracts high attention as an anticancer agent, because it is not pathogenic for humans and has oncotropic and oncosuppressive properties. The viral nonstructural NS1 protein is thought to mediate H-1PV cytotoxicity, but its exact contribution to this process remains undefined. In this study, we analyzed the effects of the H-1PV NS1 protein on human cell proliferation and cell viability. We show that NS1 expression is sufficient to induce the accumulation of cells in G(2) phase, apoptosis via caspase 9 and 3 activation, and cell lysis. Similarly, cells infected with wild-type H-1PV arrest in G(2) phase and undergo apoptosis. Furthermore, we also show that both expression of NS1 and H-1PV infection lead to higher levels of intracellular reactive oxygen species (ROS), associated with DNA double-strand breaks. Antioxidant treatment reduces ROS levels and strongly decreases NS1- and virus-induced DNA damage, cell cycle arrest, and apoptosis, indicating that NS1-induced ROS are important mediators of H-1PV cytotoxicity.
Figures




Similar articles
-
Viral Nonstructural Protein 1 Induces Mitochondrion-Mediated Apoptosis in Mink Enteritis Virus Infection.J Virol. 2019 Oct 29;93(22):e01249-19. doi: 10.1128/JVI.01249-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31484746 Free PMC article.
-
An in-frame deletion in the NS protein-coding sequence of parvovirus H-1PV efficiently stimulates export and infectivity of progeny virions.J Virol. 2012 Jul;86(14):7554-64. doi: 10.1128/JVI.00212-12. Epub 2012 May 2. J Virol. 2012. PMID: 22553326 Free PMC article.
-
Porcine Parvovirus Infection Causes Pig Placenta Tissue Damage Involving Nonstructural Protein 1 (NS1)-Induced Intrinsic ROS/Mitochondria-Mediated Apoptosis.Viruses. 2019 Apr 25;11(4):389. doi: 10.3390/v11040389. Viruses. 2019. PMID: 31027293 Free PMC article.
-
[Advances in Parvovirus Non-structural Protein NS1 Induced Apoptosis].Bing Du Xue Bao. 2015 Nov;31(6):679-84. Bing Du Xue Bao. 2015. PMID: 26951015 Review. Chinese.
-
Parvovirus B19 nonstructural (NS1) protein as a transactivator of interleukin-6 synthesis: common pathway in inflammatory sequelae of human parvovirus infections?J Med Virol. 2002 Jun;67(2):267-74. doi: 10.1002/jmv.2217. J Med Virol. 2002. PMID: 11992589 Review.
Cited by
-
Oncolytic parvoviruses: from basic virology to clinical applications.Virol J. 2015 Jan 29;12:6. doi: 10.1186/s12985-014-0223-y. Virol J. 2015. PMID: 25630937 Free PMC article. Review.
-
Oncosuppressive suicide gene virotherapy "PVH1-yCD/5-FC" for pancreatic peritoneal carcinomatosis treatment: NFκB and Akt/PI3K involvement.PLoS One. 2013 Aug 14;8(8):e70594. doi: 10.1371/journal.pone.0070594. eCollection 2013. PLoS One. 2013. PMID: 23967078 Free PMC article.
-
Retargeting of rat parvovirus H-1PV to cancer cells through genetic engineering of the viral capsid.J Virol. 2012 Apr;86(7):3452-65. doi: 10.1128/JVI.06208-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258256 Free PMC article.
-
Canine parvovirus induces G1/S cell cycle arrest that involves EGFR Tyr1086 phosphorylation.Virulence. 2020 Dec;11(1):1203-1214. doi: 10.1080/21505594.2020.1814091. Virulence. 2020. PMID: 32877289 Free PMC article.
-
Cancer Treatment Goes Viral: Using Viral Proteins to Induce Tumour-Specific Cell Death.Cancers (Basel). 2019 Dec 7;11(12):1975. doi: 10.3390/cancers11121975. Cancers (Basel). 2019. PMID: 31817939 Free PMC article. Review.
References
-
- Armstrong, J. S., and M. Whiteman. 2007. Measurement of reactive oxygen species in cells and mitochondria. Methods Cell Biol. 80:355-377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

