Rimonabant-induced Delta9-tetrahydrocannabinol withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs
- PMID: 20375197
- PMCID: PMC2912042
- DOI: 10.1124/jpet.110.168435
Rimonabant-induced Delta9-tetrahydrocannabinol withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs
Abstract
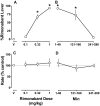
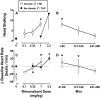
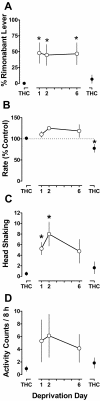
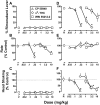
Marijuana-dependent individuals report using marijuana to alleviate withdrawal, suggesting that pharmacotherapy of marijuana withdrawal could promote abstinence. To identify potential pharmacotherapies for marijuana withdrawal, this study first characterized rimonabant-induced Delta(9)-tetrahydrocannabinol (Delta(9)-THC) withdrawal in rhesus monkeys by using drug discrimination and directly observable signs. Second, drugs were examined for their capacity to modify cannabinoid withdrawal. Monkeys receiving chronic Delta(9)-THC (1 mg/kg/12 h s.c.) discriminated the cannabinoid antagonist rimonabant (1 mg/kg i.v.) under a fixed ratio schedule of stimulus-shock termination. The discriminative stimulus effects of rimonabant were dose-dependent (ED(50) = 0.25 mg/kg) and accompanied by head shaking. In the absence of chronic Delta(9)-THC treatment (i.e., in nondependent monkeys), a larger dose (3.2 mg/kg) of rimonabant produced head shaking and tachycardia. Temporary discontinuation of Delta(9)-THC treatment resulted in increased responding on the rimonabant lever, head shaking, and activity during the dark cycle. The rimonabant discriminative stimulus was attenuated fully by Delta(9)-THC (at doses larger than mg/kg/12 h) and the cannabinoid agonist CP 55940 [5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]phenol], and partially by the cannabinoid agonist WIN 55212-2 [(R)-(+)-[2, 3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate] and the alpha(2)-adrenergic agonist clonidine. In contrast, a benzodiazepine (diazepam) and monoamine agonist (cocaine) did not attenuate the rimonabant discriminative stimulus. Head shaking was attenuated by all test compounds. These results show that the discriminative stimulus effects of rimonabant in Delta(9)-THC-treated monkeys are a more pharmacologically selective measure of cannabinoid withdrawal than rimonabant-induced head shaking. These results suggest that cannabinoid and noncannabinoid (alpha(2)-adrenergic) agonists are potentially useful therapeutics for marijuana dependence inasmuch as they attenuate the subjective experience of Delta(9)-THC withdrawal.
Figures
Similar articles
-
The cannabinoid agonist HU-210: pseudo-irreversible discriminative stimulus effects in rhesus monkeys.Eur J Pharmacol. 2014 Mar 15;727:35-42. doi: 10.1016/j.ejphar.2014.01.041. Epub 2014 Jan 30. Eur J Pharmacol. 2014. PMID: 24486701 Free PMC article.
-
Discriminative stimulus effects of the cannabinoid CB1 antagonist SR 141716A in rhesus monkeys pretreated with Delta9-tetrahydrocannabinol.Psychopharmacology (Berl). 2006 Oct;188(3):306-14. doi: 10.1007/s00213-006-0500-6. Epub 2006 Sep 5. Psychopharmacology (Berl). 2006. PMID: 16953389
-
Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Delta9-tetrahydrocannabinol.J Pharmacol Exp Ther. 2006 Dec;319(3):1211-8. doi: 10.1124/jpet.106.107110. Epub 2006 Aug 30. J Pharmacol Exp Ther. 2006. PMID: 16943255
-
Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists.Curr Med Chem. 2010;17(14):1360-81. doi: 10.2174/092986710790980050. Curr Med Chem. 2010. PMID: 20166927 Free PMC article. Review.
-
Use of dronabinol for cannabis dependence: two case reports and review.Am J Addict. 2008 Mar-Apr;17(2):161-4. doi: 10.1080/10550490701861177. Am J Addict. 2008. PMID: 18393061 Free PMC article. Review.
Cited by
-
Chronic Δ9-THC in Rhesus Monkeys: Effects on Cognitive Performance and Dopamine D2/D3 Receptor Availability.J Pharmacol Exp Ther. 2018 Feb;364(2):300-310. doi: 10.1124/jpet.117.244194. Epub 2017 Dec 4. J Pharmacol Exp Ther. 2018. PMID: 29203575 Free PMC article.
-
JWH-018 and JWH-073: Δ⁹-tetrahydrocannabinol-like discriminative stimulus effects in monkeys.J Pharmacol Exp Ther. 2012 Jan;340(1):37-45. doi: 10.1124/jpet.111.187757. Epub 2011 Sep 30. J Pharmacol Exp Ther. 2012. PMID: 21965552 Free PMC article.
-
Effects of continuous nicotine treatment and subsequent termination on cocaine versus food choice in male rhesus monkeys.Exp Clin Psychopharmacol. 2015 Oct;23(5):395-404. doi: 10.1037/pha0000023. Epub 2015 Jun 22. Exp Clin Psychopharmacol. 2015. PMID: 26098473 Free PMC article.
-
Functional consequences of short-term exposure to opioids versus cannabinoids in nonhuman primates.Neuropharmacology. 2023 Feb 1;223:109328. doi: 10.1016/j.neuropharm.2022.109328. Epub 2022 Nov 8. Neuropharmacology. 2023. PMID: 36356937 Free PMC article.
-
The cannabinoid agonist HU-210: pseudo-irreversible discriminative stimulus effects in rhesus monkeys.Eur J Pharmacol. 2014 Mar 15;727:35-42. doi: 10.1016/j.ejphar.2014.01.041. Epub 2014 Jan 30. Eur J Pharmacol. 2014. PMID: 24486701 Free PMC article.
References
-
- Aceto MD, Scates SM, Lowe JA, Martin BR. (1996) Dependence on Δ9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. J Pharmacol Exp Ther 278:1290–1295 - PubMed
-
- Beardsley PM, Balster RL, Harris LS. (1986) Dependence on tetrahydrocannabinol in rhesus monkeys. J Pharmacol Exp Ther 239:311–319 - PubMed
-
- Boisse NR, Okamoto M. (1978) Physical dependence to barbital compared to pentobarbital. IV. Influence of elimination kinetics. J Pharmacol Exp Ther 204:526–540 - PubMed
-
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, et al. (1997) A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem 272:22330–22339 - PubMed
-
- Budney AJ, Hughes JR, Moore BA, Vandrey R. (2004) Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry 161:1967–1977 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

