Stomatal vs. genome size in angiosperms: the somatic tail wagging the genomic dog?
- PMID: 20375204
- PMCID: PMC2850795
- DOI: 10.1093/aob/mcq011
Stomatal vs. genome size in angiosperms: the somatic tail wagging the genomic dog?
Abstract
Background and aims: Genome size is a function, and the product, of cell volume. As such it is contingent on ecological circumstance. The nature of 'this ecological circumstance' is, however, hotly debated. Here, we investigate for angiosperms whether stomatal size may be this 'missing link': the primary determinant of genome size. Stomata are crucial for photosynthesis and their size affects functional efficiency.
Methods: Stomatal and leaf characteristics were measured for 1442 species from Argentina, Iran, Spain and the UK and, using PCA, some emergent ecological and taxonomic patterns identified. Subsequently, an assessment of the relationship between genome-size values obtained from the Plant DNA C-values database and measurements of stomatal size was carried out.
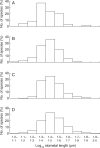
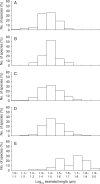
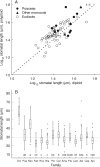
Key results: Stomatal size is an ecologically important attribute. It varies with life-history (woody species < herbaceous species < vernal geophytes) and contributes to ecologically and physiologically important axes of leaf specialization. Moreover, it is positively correlated with genome size across a wide range of major taxa.
Conclusions: Stomatal size predicts genome size within angiosperms. Correlation is not, however, proof of causality and here our interpretation is hampered by unexpected deficiencies in the scientific literature. Firstly, there are discrepancies between our own observations and established ideas about the ecological significance of stomatal size; very large stomata, theoretically facilitating photosynthesis in deep shade, were, in this study (and in other studies), primarily associated with vernal geophytes of unshaded habitats. Secondly, the lower size limit at which stomata can function efficiently, and the ecological circumstances under which these minute stomata might occur, have not been satisfactorally resolved. Thus, our hypothesis, that the optimization of stomatal size for functional efficiency is a major ecological determinant of genome size, remains unproven.
Figures


Similar articles
-
Genome size is a strong predictor of cell size and stomatal density in angiosperms.New Phytol. 2008;179(4):975-986. doi: 10.1111/j.1469-8137.2008.02528.x. Epub 2008 Jun 28. New Phytol. 2008. PMID: 18564303
-
Leaf surface development and the plant fossil record: stomatal patterning in Bennettitales.Biol Rev Camb Philos Soc. 2019 Jun;94(3):1179-1194. doi: 10.1111/brv.12497. Epub 2019 Feb 4. Biol Rev Camb Philos Soc. 2019. PMID: 30714286 Review.
-
Environmental pressures on stomatal size may drive plant genome size evolution: evidence from a natural experiment with Cape geophytes.Ann Bot. 2020 Jul 24;126(2):323-330. doi: 10.1093/aob/mcaa095. Ann Bot. 2020. PMID: 32474609 Free PMC article.
-
Ultrastructure of stomatal development in early-divergent angiosperms reveals contrasting patterning and pre-patterning.Ann Bot. 2013 Oct;112(6):1031-43. doi: 10.1093/aob/mct169. Epub 2013 Aug 21. Ann Bot. 2013. PMID: 23969762 Free PMC article.
-
Stomatal development in time: the past and the future.Curr Opin Genet Dev. 2017 Aug;45:1-9. doi: 10.1016/j.gde.2017.02.001. Epub 2017 Feb 20. Curr Opin Genet Dev. 2017. PMID: 28219014 Review.
Cited by
-
Genome size of alpine plants does not predict temperature resistance.Planta. 2022 Jun 24;256(1):18. doi: 10.1007/s00425-022-03935-x. Planta. 2022. PMID: 35748952
-
Trade-offs between seed and leaf size (seed-phytomer-leaf theory): functional glue linking regenerative with life history strategies … and taxonomy with ecology?Ann Bot. 2017 Nov 10;120(5):633-652. doi: 10.1093/aob/mcx084. Ann Bot. 2017. PMID: 28961937 Free PMC article.
-
Divergent Adaptive Strategies by Two Co-occurring Epiphytic Orchids to Water Stress: Escape or Avoidance?Front Plant Sci. 2016 May 3;7:588. doi: 10.3389/fpls.2016.00588. eCollection 2016. Front Plant Sci. 2016. PMID: 27200059 Free PMC article.
-
Comparative analysis of two Korean irises (Iris ruthenica and I. uniflora, Iridaceae) based on plastome sequencing and micromorphology.Sci Rep. 2022 Jun 8;12(1):9424. doi: 10.1038/s41598-022-13528-z. Sci Rep. 2022. PMID: 35676304 Free PMC article.
-
Leaf photosynthetic rate of tropical ferns is evolutionarily linked to water transport capacity.PLoS One. 2014 Jan 9;9(1):e84682. doi: 10.1371/journal.pone.0084682. eCollection 2014. PLoS One. 2014. PMID: 24416265 Free PMC article.
References
-
- Aasamaa K, Sober A, Rahi M. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Australian Journal of Plant Physiology. 2001;28:765–774.
-
- Allen MT, Pearcy RW. Stomatal behavior and photosynthetic performance under dynamic light regimes in a seasonally dry tropical rain forest. Oecologia. 2000;122:470–478. - PubMed
-
- APG III. An update of the Angiosperm Phylogeny Group Classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121.
-
- Aronne G, De Micco V. Seasonal dimorphism in the Mediterranean Cistus incanus. Annals of Botany. 2001;87:789–794.
-
- Barrow M, Meister A. Endopolyploidy in seed plants is differently correlated to systematic, organ, life strategy and genome size. Plant, Cell & Environment. 2003;26:571–584.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

