Frequency-dependent spatiotemporal tuning properties of non-eye movement related vestibular neurons to three-dimensional translations in squirrel monkeys
- PMID: 20375245
- PMCID: PMC2888244
- DOI: 10.1152/jn.00904.2009
Frequency-dependent spatiotemporal tuning properties of non-eye movement related vestibular neurons to three-dimensional translations in squirrel monkeys
Abstract
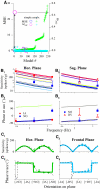
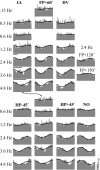
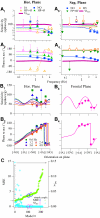
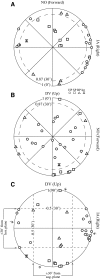
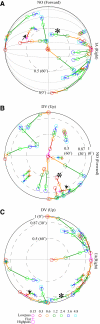
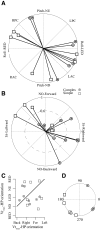
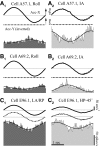
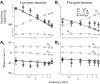
Responses of vestibular-only translation sensitive (VOTS) neurons in vestibular nuclei of two squirrel monkeys were studied at multiple frequencies to three-dimensional translations and rotations. A novel frequency-dependent spatiotemporal analysis examined in each neuron whether complex models, with unrestricted response dynamics in three-dimensional (3D) space, provided significantly better fits than restricted models following simple, cosine rule. Subsequently, the statistically selected optimal model was used to predict the maximum translation direction, expressed as a unitary vector, Vt(max), and its associated sensitivity and phase across frequencies. Simple models were sufficient to quantify the 3D translational responses of 66% of neurons. Most VOTS neurons, complex or simple, exhibited flat-gain or low-pass response dynamics. The Vt(max) of simple neurons was fixed, whereas that of complex neurons changed with frequency. The spatial distribution of Vt(max) in simple neurons, which fell within 30 degrees of either the horizontal plane or/and the sagittal plane, was closely aligned with Vt(max) of vestibular afferents. In contrast, the frequency-dependent Vt(max) of most complex neurons migrated from the dorsoventral axis at higher frequency toward the horizontal plane, especially the interaural axis, at lower frequency. When the maximum rotation direction was estimated from responses of the same VOTS neurons to 1.2 Hz yaw, pitch, and roll rotations, complex neurons were more likely to respond to rotations activating vertical canals. Responses to 0.15-0.3 Hz linear accelerations produced by inertial or gravitational forces were indistinguishable in most complex neurons but significantly different in most simple neurons. These observations suggest that simple and complex VOTS neurons constitute distinctive vestibular pathways where complex neurons, exhibiting a novel spatiotemporal filtering mechanism in processing otolith-related signals, are well suited to drive tilt-related responses, whereas simple neurons probably mediate pure translation related responses.
Figures


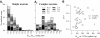
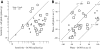
Similar articles
-
Three dimensional spatial-temporal convergence of otolith related signals in vestibular only neurons in squirrel monkeys.Exp Brain Res. 2006 Jan;168(3):410-26. doi: 10.1007/s00221-005-0098-7. Epub 2005 Sep 29. Exp Brain Res. 2006. PMID: 16193271
-
Vestibular convergence patterns in vestibular nuclei neurons of alert primates.J Neurophysiol. 2002 Dec;88(6):3518-33. doi: 10.1152/jn.00518.2002. J Neurophysiol. 2002. PMID: 12466465
-
Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses.J Neurophysiol. 2000 Oct;84(4):2113-32. doi: 10.1152/jn.2000.84.4.2113. J Neurophysiol. 2000. PMID: 11024100
-
Gravity or translation: central processing of vestibular signals to detect motion or tilt.J Vestib Res. 2003;13(4-6):245-53. J Vestib Res. 2003. PMID: 15096668 Review.
-
Bilateral otolith contribution to spatial coding in the vestibular system.J Biomed Sci. 2002 Nov-Dec;9(6 Pt 2):574-86. doi: 10.1159/000067284. J Biomed Sci. 2002. PMID: 12432223 Review.
Cited by
-
Convergence of linear acceleration and yaw rotation signals on non-eye movement neurons in the vestibular nucleus of macaques.J Neurophysiol. 2018 Jan 1;119(1):73-83. doi: 10.1152/jn.00382.2017. Epub 2017 Oct 4. J Neurophysiol. 2018. PMID: 28978765 Free PMC article.
-
Responses of non-eye movement central vestibular neurons to sinusoidal horizontal translation in compensated macaques after unilateral labyrinthectomy.J Neurophysiol. 2014 Jul 1;112(1):9-21. doi: 10.1152/jn.00748.2013. Epub 2014 Apr 9. J Neurophysiol. 2014. PMID: 24717349 Free PMC article.
-
Adaptation of spatio-temporal convergent properties in central vestibular neurons in monkeys.Physiol Rep. 2018 Sep;6(17):e13750. doi: 10.14814/phy2.13750. Physiol Rep. 2018. PMID: 30178612 Free PMC article.
-
Diversity of vestibular nuclei neurons targeted by cerebellar nodulus inhibition.J Physiol. 2014 Jan 1;592(1):171-88. doi: 10.1113/jphysiol.2013.259614. Epub 2013 Oct 14. J Physiol. 2014. PMID: 24127616 Free PMC article.
-
Neuronal thresholds and choice-related activity of otolith afferent fibers during heading perception.Proc Natl Acad Sci U S A. 2015 May 19;112(20):6467-72. doi: 10.1073/pnas.1507402112. Epub 2015 May 4. Proc Natl Acad Sci U S A. 2015. PMID: 25941358 Free PMC article.
References
-
- Angelaki DE. Spatio-temporal convergence (STC) in otolith neurons. Biol Cybern 67: 83–96, 1992 - PubMed
-
- Angelaki DE. Spatial and temporal coding in single neurons. Biol Cybern 69: 147–154, 1993 - PubMed
-
- Angelaki DE. Three-dimensional organization of otolith-ocular reflexes in rhesus monkeys. III. Responses to translation. J Neurophysiol 80: 680–695, 1998 - PubMed
-
- Angelaki DE, Bush GA, Perachio AA. A model for the characterization of the spatial properties in vestibular neurons. Biol Cybern 66: 231–240, 1992 - PubMed
-
- Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol 84: 2113–2132, 2000 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

