Properties of GABAergic neurons in the rostral solitary tract nucleus in mice
- PMID: 20375246
- PMCID: PMC2888243
- DOI: 10.1152/jn.00971.2009
Properties of GABAergic neurons in the rostral solitary tract nucleus in mice
Abstract
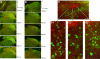
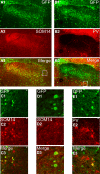
The rostral nucleus of the solitary tract (rNST) plays a pivotal role in taste processing. The rNST contains projection neurons and interneurons that differ in morphology and intrinsic membrane properties. Although characteristics of the projection neurons have been detailed, similar information is lacking on the interneurons. We determined the intrinsic properties of the rNST GABAergic interneurons using a transgenic mouse model that expresses enhanced green fluorescent protein under the control of a GAD67 promoter. Glutamic acid decarboxylase-green fluorescent protein (GAD67-GFP) neurons were distributed throughout the rNST but were concentrated in the ventral subdivision with minimal interaction with the terminal field of the afferent input. Furthermore, the density of the GAD67-GFP neurons decreased in more rostral areas of rNST. In whole cell recordings, GAD67-GFP neurons responded with either an initial burst (73%), tonic (18%), or irregular (9%) discharge pattern of action potentials (APs) in response to membrane depolarization. These three groups also differed in passive and AP characteristics. Initial burst neurons had small ovoid or fusiform cell bodies, whereas tonic firing neurons had large multipolar or fusiform cell bodies. Irregular firing neurons had larger spherical soma. Some of the initial burst and tonic firing neurons were also spontaneously active. The GAD67-GFP neurons could also be categorized in subgroups based on colocalization with somatostatin and parvalbumin immunolabeling. Initial burst neurons would transmit the early dynamic portion of the encoded sensory stimuli, whereas tonic firing neurons could respond to both dynamic and static components of the sensory input, suggesting different roles for GAD67-GFP neurons in taste processing.
Figures






References
-
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS). J Neurophysiol 99: 1712–1722, 2008 - PubMed
-
- Barry MA, Halsell CB, Whitehead MC. Organization of the nucleus of the solitary tract in the hamster: acetylcholinesterase, NADH dehydrogenase, and cytochrome oxidase histochemistry. Microsc Res Tech 26: 231–244, 1993 - PubMed
-
- Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res 557: 265–279, 1991 - PubMed
-
- Bradley RM. The Role of the Nucleus of the Solitary Tract in Gustatory Processing Boca Raton, FL: CRC Press, 2006 - PubMed
-
- Bradley RM, Sweazey RD. Separation of neuron types in the gustatory zone of the nucleus tractus solitarii based on intrinsic firing properties. J Neurophysiol 67: 1659–1668, 1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

