Ovarian furin (proprotein convertase subtilisin/kexin type3): expression, localization, and potential role in ovulation in the rat
- PMID: 20375258
- PMCID: PMC2888968
- DOI: 10.1095/biolreprod.109.079947
Ovarian furin (proprotein convertase subtilisin/kexin type3): expression, localization, and potential role in ovulation in the rat
Abstract
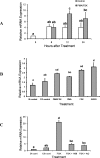
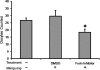
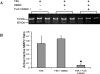
The process of ovulation involves weakening of the follicular wall by proteolytic enzymes. The function of FURIN (also known as PCSK3) is to activate various proteolytic enzymes. In the present study, the expression, localization, and function of FURIN were investigated in the periovulatory rat ovary. Immature female rats were injected with equine chorionic gonadotropin followed by human chorionic gonadotropin (hCG) 48 h later to stimulate ovulation. Ovaries were collected at 0, 4, 8, 12, and 24 h after hCG injection. Administration of hCG increased Furin mRNA expression in both intact ovaries and cultured ovarian follicles to maximal levels at 8 and 12 h before decreasing at 24 h. In cultured granulosa cells, Furin mRNA levels were significantly induced at 12 h after hCG. In situ hybridization of Furin mRNA demonstrated expression in the granulosa cells, with predominant expression in the theca layer. Regulation studies demonstrated that Furin mRNA was induced in residual tissue by forskolin or amphiregulin. To examine the role of FURIN in protease activation and ovulation, rats were treated with a FURIN inhibitor and oocyte release was determined. There was a 38% decrease in the number of oocytes released in ovaries treated with the FURIN inhibitor. Likewise, the FURIN inhibitor decreased the activation of MMP2. The induction of Furin mRNA after treatment with hCG, along with the decrease in MMP2 activation and oocyte release after FURIN inhibition, supports the hypothesis that FURIN is upregulated during the preovulatory period, which results in activation of proteinases associated with the breakdown of the follicular wall during ovulation.
Figures


Similar articles
-
Hormonal regulation of proprotein convertase subtilisin/kexin type 5 expression during ovarian follicle development in the rat.Mol Cell Endocrinol. 2008 Jul 16;289(1-2):29-37. doi: 10.1016/j.mce.2008.04.006. Epub 2008 Apr 22. Mol Cell Endocrinol. 2008. PMID: 18502031
-
Cellular localization of gelatinases and tissue inhibitors of metalloproteinases during follicular growth, ovulation, and early luteal formation in the rat.Biol Reprod. 2001 Sep;65(3):855-65. doi: 10.1095/biolreprod65.3.855. Biol Reprod. 2001. PMID: 11514351
-
Membrane type 1-matrix metalloproteinase (MMP)-associated MMP-2 activation increases in the rat ovary in response to an ovulatory dose of human chorionic gonadotropin.Biol Reprod. 2004 Apr;70(4):1024-32. doi: 10.1095/biolreprod.103.023499. Epub 2003 Dec 10. Biol Reprod. 2004. PMID: 14668206
-
Ovarian expression and regulation of the stromelysins during the periovulatory period in the human and the rat.Biol Reprod. 2012 Mar 22;86(3):78. doi: 10.1095/biolreprod.111.095588. Print 2012 Mar. Biol Reprod. 2012. PMID: 22116802 Free PMC article.
-
MMPS and TIMPS in ovarian physiology and pathophysiology.Front Biosci. 2004 Sep 1;9:2474-83. doi: 10.2741/1409. Front Biosci. 2004. PMID: 15353300 Review.
Cited by
-
Relative expression of proprotein convertases in rat ovaries during pregnancy.J Ovarian Res. 2013 Dec 11;6(1):91. doi: 10.1186/1757-2215-6-91. J Ovarian Res. 2013. PMID: 24330629 Free PMC article.
-
Ovarian granulosa cells from women with PCOS express low levels of SARS-CoV-2 receptors and co-factors.Arch Gynecol Obstet. 2022 Aug;306(2):547-555. doi: 10.1007/s00404-022-06567-4. Epub 2022 Apr 27. Arch Gynecol Obstet. 2022. PMID: 35477803 Free PMC article.
-
An ex vivo ovulation system enables the discovery of novel ovulatory pathways and nonhormonal contraceptive candidates†.Biol Reprod. 2023 Apr 11;108(4):629-644. doi: 10.1093/biolre/ioad009. Biol Reprod. 2023. PMID: 36708230 Free PMC article.
-
Proprotein convertase furin regulates apoptosis and proliferation of granulosa cells in the rat ovary.PLoS One. 2013;8(2):e50479. doi: 10.1371/journal.pone.0050479. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418414 Free PMC article.
-
Immunohistochemical expression of MMP-14 and MMP-2, and MMP-2 activity during human ovarian follicular development.Reprod Biol Endocrinol. 2014 Jan 31;12:12. doi: 10.1186/1477-7827-12-12. Reprod Biol Endocrinol. 2014. PMID: 24485069 Free PMC article.
References
-
- Curry TE, Jr, Osteen KG.The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: 428–465. - PubMed
-
- Curry TE, Jr, Dean DD, Woessner JF, Jr, LeMaire WJ.The extraction of a tissue collagenase associated with ovulation in the rat. Biol Reprod 1985; 33: 981–991. - PubMed
-
- Kaur C, Guraya SS.Effects of blocking ovulation with pentobarbitone on ovarian proteinases in rats. Curr Sci 1986; 55: 995–996.
-
- Reich R, Tsafriri A, Mechanic GL.The involvement of collagenolysis in ovulation in the rat. Endocrinology 1985; 116: 522–527. - PubMed
-
- Kleiner DE, Jr, Stetler-Stevenson WG.Structural biochemistry and activation of matrix metalloproteases. Curr Opin Cell Biol 1993; 5: 891–897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

