Effect of chronic contractile activity on mRNA stability in skeletal muscle
- PMID: 20375275
- PMCID: PMC2904257
- DOI: 10.1152/ajpcell.00523.2009
Effect of chronic contractile activity on mRNA stability in skeletal muscle
Abstract
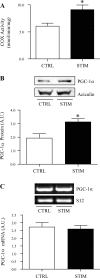
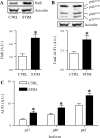
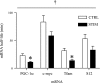
Repeated bouts of exercise promote the biogenesis of mitochondria by multiple steps in the gene expression patterning. The role of mRNA stability in controlling the expression of mitochondrial proteins is relatively unexplored. To induce mitochondrial biogenesis, we chronically stimulated (10 Hz; 3 or 6 h/day) rat muscle for 7 days. Chronic contractile activity (CCA) increased the protein expression of PGC-1alpha, c-myc, and mitochondrial transcription factor A (Tfam) by 1.6-, 1.7- and 2.0-fold, respectively. To determine mRNA stability, we incubated total RNA with cytosolic extracts using an in vitro cell-free system. We found that the intrinsic mRNA half-lives (t(1/2)) were variable within control muscle. Peroxisome proliferator-activated receptor-gamma, coactivator-1alpha (PGC-1alpha) and Tfam mRNAs decayed more rapidly (t(1/2) = 22.7 and 31.4 min) than c-myc mRNA (t(1/2) = 99.7 min). Furthermore, CCA resulted in a differential response in degradation kinetics. After CCA, PGC-1alpha and Tfam mRNA half-lives decreased by 48% and 44%, respectively, whereas c-myc mRNA half-life was unchanged. CCA induced an elevation of both the cytosolic RNA-stabilizing human antigen R (HuR) and destabilizing AUF1 (total) by 2.4- and 1.8-fold, respectively. Increases in the p37(AUF1), p40(AUF1), and p45(AUF1) isoforms were most evident. Thus these data indicate that CCA results in accelerated turnover rates of mRNAs encoding important mitochondrial biogenesis regulators in skeletal muscle. This adaptation is likely beneficial in permitting more rapid phenotypic plasticity in response to subsequent contractile activity.
Figures




Similar articles
-
Exercise training increases the expression and nuclear localization of mRNA destabilizing proteins in skeletal muscle.Am J Physiol Regul Integr Comp Physiol. 2013 Oct 1;305(7):R822-31. doi: 10.1152/ajpregu.00590.2012. Epub 2013 Jul 31. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23904104
-
mRNA stability as a function of striated muscle oxidative capacity.Am J Physiol Regul Integr Comp Physiol. 2012 Aug 15;303(4):R408-17. doi: 10.1152/ajpregu.00085.2012. Epub 2012 Jun 20. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22718808
-
Interaction between 3' untranslated region of calcitonin receptor messenger ribonucleic acid (RNA) and adenylate/uridylate (AU)-rich element binding proteins (AU-rich RNA-binding factor 1 and Hu antigen R).Endocrinology. 2004 Apr;145(4):1730-8. doi: 10.1210/en.2003-0862. Epub 2004 Jan 8. Endocrinology. 2004. PMID: 14715706
-
Polyamines regulate the stability of JunD mRNA by modulating the competitive binding of its 3' untranslated region to HuR and AUF1.Mol Cell Biol. 2010 Nov;30(21):5021-32. doi: 10.1128/MCB.00807-10. Epub 2010 Aug 30. Mol Cell Biol. 2010. PMID: 20805360 Free PMC article.
-
Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence.Biol Chem. 2008 Mar;389(3):243-55. doi: 10.1515/BC.2008.022. Biol Chem. 2008. PMID: 18177264 Free PMC article. Review.
Cited by
-
Review of the current and potential use of biological and molecular methods for the estimation of the postmortem interval in animals and humans.J Vet Diagn Invest. 2023 Mar;35(2):97-108. doi: 10.1177/10406387231153930. Epub 2023 Feb 6. J Vet Diagn Invest. 2023. PMID: 36744749 Free PMC article. Review.
-
Aging Hallmarks: The Benefits of Physical Exercise.Front Endocrinol (Lausanne). 2018 May 25;9:258. doi: 10.3389/fendo.2018.00258. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29887832 Free PMC article. Review.
-
Exercise attenuates the major hallmarks of aging.Rejuvenation Res. 2015 Feb;18(1):57-89. doi: 10.1089/rej.2014.1623. Rejuvenation Res. 2015. PMID: 25431878 Free PMC article. Review.
-
Modulation of neoplastic gene regulatory pathways by the RNA-binding factor AUF1.Front Biosci (Landmark Ed). 2011 Jun 1;16(6):2307-25. doi: 10.2741/3855. Front Biosci (Landmark Ed). 2011. PMID: 21622178 Free PMC article. Review.
-
Effects of exercise on cellular and tissue aging.Aging (Albany NY). 2021 May 13;13(10):14522-14543. doi: 10.18632/aging.203051. Epub 2021 May 13. Aging (Albany NY). 2021. PMID: 34001677 Free PMC article. Review.
References
-
- Adhihetty PJ, Ljubicic V, Hood DA. Effect of chronic contractile activity on SS and IMF mitochondrial apoptotic susceptibility in skeletal muscle. Am J Physiol Endocrinol Metab 292: E748–E755, 2007 - PubMed
-
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280: 19587–19593, 2005 - PubMed
-
- Aoi W, Naito Y, Mizushima K, Takanami Y, Kawai Y, Ichikawa H, Yoshikawa T. The microRNA miR-696 regulates PGC1α in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab 298: E799–E806, 2010 - PubMed
-
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5: 35–46, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

