Temporal delays among place cells determine the frequency of population theta oscillations in the hippocampus
- PMID: 20375279
- PMCID: PMC2867922
- DOI: 10.1073/pnas.0912478107
Temporal delays among place cells determine the frequency of population theta oscillations in the hippocampus
Abstract
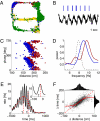
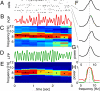
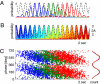
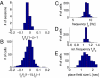
Driven either by external landmarks or by internal dynamics, hippocampal neurons form sequences of cell assemblies. The coordinated firing of these active cells is organized by the prominent "theta" oscillations in the local field potential (LFP): place cells discharge at progressively earlier theta phases as the rat crosses the respective place field ("phase precession"). The faster oscillation frequency of active neurons and the slower theta LFP, underlying phase precession, creates a paradox. How can faster oscillating neurons comprise a slower population oscillation, as reflected by the LFP? We built a mathematical model that allowed us to calculate the population activity analytically from experimentally derived parameters of the single neuron oscillation frequency, firing field size (duration), and the relationship between within-theta delays of place cell pairs and their distance representations ("compression"). The appropriate combination of these parameters generated a constant frequency population rhythm along the septo-temporal axis of the hippocampus, while allowing individual neurons to vary their oscillation frequency and field size. Our results suggest that the faster-than-theta oscillations of pyramidal cells are inherent and that phase precession is a result of the coordinated activity of temporally shifted cell assemblies, relative to the population activity, reflected by the LFP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials.Hippocampus. 1998;8(3):244-61. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. Hippocampus. 1998. PMID: 9662139
-
Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences.Hippocampus. 1996;6(2):149-72. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. Hippocampus. 1996. PMID: 8797016
-
A temporal mechanism for generating the phase precession of hippocampal place cells.J Comput Neurosci. 2000 Jul-Aug;9(1):5-30. doi: 10.1023/a:1008976210366. J Comput Neurosci. 2000. PMID: 10946990
-
Theta phase precession beyond the hippocampus.Rev Neurosci. 2012;23(1):39-65. doi: 10.1515/revneuro-2011-0064. Rev Neurosci. 2012. PMID: 22718612 Review.
-
The theta/gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme.Hippocampus. 2005;15(7):913-22. doi: 10.1002/hipo.20121. Hippocampus. 2005. PMID: 16161035 Review.
Cited by
-
Efficient phase coding in hippocampal place cells.Phys Rev Res. 2020 Sep 11;2(3):033393. doi: 10.1103/PhysRevResearch.2.033393. eCollection 2020 Jul-Sep. Phys Rev Res. 2020. PMID: 32984841 Free PMC article.
-
The neural circuitry supporting successful spatial navigation despite variable movement speeds.Neurosci Biobehav Rev. 2020 Jan;108:821-833. doi: 10.1016/j.neubiorev.2019.11.013. Epub 2019 Nov 21. Neurosci Biobehav Rev. 2020. PMID: 31760048 Free PMC article. Review.
-
Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference.J Neurosci. 2011 Nov 9;31(45):16157-76. doi: 10.1523/JNEUROSCI.0712-11.2011. J Neurosci. 2011. PMID: 22072668 Free PMC article.
-
Flexible theta sequence compression mediated via phase precessing interneurons.Elife. 2016 Dec 8;5:e20349. doi: 10.7554/eLife.20349. Elife. 2016. PMID: 27929374 Free PMC article.
-
Independent theta phase coding accounts for CA1 population sequences and enables flexible remapping.Elife. 2015 Feb 2;4:e03542. doi: 10.7554/eLife.03542. Elife. 2015. PMID: 25643396 Free PMC article.
References
-
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. - PubMed
-
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. - PubMed
-
- Petsche H, Stumpf C, Gogolak G. [The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippo-campus arousal activity by the septum cells] Electroencephalogr Clin Neurophysiol. 1962;14:202–211. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

