Attenuated activation of macrophage TLR9 by DNA from virulent mycobacteria
- PMID: 20375564
- PMCID: PMC7312848
- DOI: 10.1159/000142731
Attenuated activation of macrophage TLR9 by DNA from virulent mycobacteria
Abstract
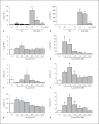
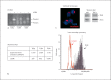
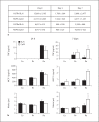
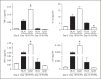
Alveolar macrophages are the first line of host defence against mycobacteria, but an insufficient host response allows survival of bacteria within macrophages. We aimed to investigate the role of Toll-like receptor 9 (TLR9) activation in macrophage defence against mycobacteria. Human in vitro differentiated macrophages as well as human and mouse alveolar macrophages showed TLR9 mRNA and protein expression. The cells were markedly activated by DNA isolated from attenuated mycobacterial strains (H37Ra and Mycobacterium bovis BCG) as assessed by measuring cytokine expression by real-time PCR, whereas synthetic phosphorothioate-modified oligonucleotides had a much lower potency to activate human macrophages. Intracellular replication of H37Ra was higher in macrophages isolated from TLR9-deficient mice than in macrophages from wild-type mice, whereas H37Rv showed equal survival in cells from wild-type or mutant mice. Increased bacterial survival in mouse macrophages was accompanied by altered cytokine production as determined by Luminex bead assays. In vivo infection experiments also showed differential cytokine production in TLR9-deficient mice compared to wild-type animals. Both human monocyte-derived macrophages as well as human alveolar macrophages showed reduced activation upon treatment with DNA isolated from bacteria from virulent (M. bovis and H37Rv) compared to attenuated mycobacteria. We suggest attenuated TLR9 activation contributes to the insufficient host response against virulent mycobacteria.
Copyright 2008 S. Karger AG, Basel.
Figures


References
-
- Doffinger R, Patel SY, Kumararatne DS. Host genetic factors and mycobacterial infections: lessons from single gene disorders affecting innate and adaptive immunity. Microbes Infect. 2006;8:1141–1150. - PubMed
-
- Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. - PubMed
-
- Shimada S, Yano O, Inoue H, Kuramoto E, Fukuda T, Yamamoto H, Kataoka T, Tokunaga T. Antitumor activity of the DNA fraction from Mycobacterium bovis BCG. II. Effects on various syngeneic mouse tumors. J Natl Cancer Inst. 1985;74:681–688. - PubMed
-
- Tokunaga T, Yamamoto H, Shimada S, Abe H, Fukuda T, Fujisawa Y, Furutani Y, Yano O, Kataoka T, Sudo T. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984;72:955–962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

