A new pathway of staphylococcal pathogenesis: apoptosis-like death induced by Staphopain B in human neutrophils and monocytes
- PMID: 20375568
- PMCID: PMC7312767
- DOI: 10.1159/000181014
A new pathway of staphylococcal pathogenesis: apoptosis-like death induced by Staphopain B in human neutrophils and monocytes
Abstract
Circulating neutrophils and monocytes form the first line of cellular defense against invading bacteria. Here, we describe a novel and specific mechanism of disabling and eliminating phagocytes by Staphylococcus aureus. Staphopain B (SspB) selectively cleaved CD11b on phagocytes, which rapidly acquired features of cell death. SspB-treated phagocytes expressed phosphatidylserine as well as annexin I and became permeable to propidium iodide, thus demonstrating distinctive features of both apoptosis and necrosis, respectively. The cell death observed was caspase and Syk tyrosine kinase independent, whilst cytochalasin D efficiently inhibited the staphopain-induced neutrophil killing. Neutrophil and monocyte cell death was not affected by integrin clustering ligands (ICAM-1 or fibrin) and was prevented, and even reversed, by IgG. This protective effect was dependent on the Fc fragment, collectively suggesting cooperation of the CD16 receptor and integrin Mac-1 (CD11b/CD18). We conclude that SspB, particularly in the presence of staphylococcal protein A, may reduce the number of functional phagocytes at infection sites, thus facilitating colonization and dissemination of S. aureus.
Copyright 2008 S. Karger AG, Basel.
Figures

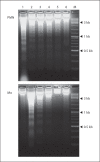
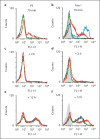

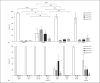

Comment in
-
Bacterial proteases disarming host defense.J Innate Immun. 2009;1(2):69. doi: 10.1159/000181143. Epub 2008 Dec 2. J Innate Immun. 2009. PMID: 20375567 Free PMC article. No abstract available.
Similar articles
-
Staphylococcal cysteine protease staphopain B (SspB) induces rapid engulfment of human neutrophils and monocytes by macrophages.Biol Chem. 2009 Apr;390(4):361-71. doi: 10.1515/BC.2009.042. Biol Chem. 2009. PMID: 19284294
-
Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1.Proc Natl Acad Sci U S A. 2013 Jun 25;110(26):10794-9. doi: 10.1073/pnas.1305121110. Epub 2013 Jun 10. Proc Natl Acad Sci U S A. 2013. PMID: 23754403 Free PMC article.
-
Complement Receptor 3 Contributes to the Sexual Dimorphism in Neutrophil Killing of Staphylococcus aureus.J Immunol. 2020 Sep 15;205(6):1593-1600. doi: 10.4049/jimmunol.2000545. Epub 2020 Aug 7. J Immunol. 2020. PMID: 32769122 Free PMC article.
-
Defense against own arms: staphylococcal cysteine proteases and their inhibitors.Acta Biochim Pol. 2003;50(3):715-24. Acta Biochim Pol. 2003. PMID: 14515151 Review.
-
Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus.Front Cell Infect Microbiol. 2017 Jun 30;7:286. doi: 10.3389/fcimb.2017.00286. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713774 Free PMC article. Review.
Cited by
-
Developing phytocompound-based new drugs against multi-drug-resistant Staphylococcus aureus.R Soc Open Sci. 2024 Jul 24;11(7):231475. doi: 10.1098/rsos.231475. eCollection 2024 Jul. R Soc Open Sci. 2024. PMID: 39050719 Free PMC article.
-
Staphylococcus aureus Secreted Toxins and Extracellular Enzymes.Microbiol Spectr. 2019 Mar;7(2):10.1128/microbiolspec.gpp3-0039-2018. doi: 10.1128/microbiolspec.GPP3-0039-2018. Microbiol Spectr. 2019. PMID: 30873936 Free PMC article. Review.
-
Rapid decrease of CD16 (FcγRIII) expression on heat-shocked neutrophils and their recognition by macrophages.J Biomed Biotechnol. 2011;2011:284759. doi: 10.1155/2011/284759. Epub 2011 Apr 27. J Biomed Biotechnol. 2011. PMID: 21541219 Free PMC article.
-
Intracellular Staphylococcus aureus employs the cysteine protease staphopain A to induce host cell death in epithelial cells.PLoS Pathog. 2021 Sep 2;17(9):e1009874. doi: 10.1371/journal.ppat.1009874. eCollection 2021 Sep. PLoS Pathog. 2021. PMID: 34473800 Free PMC article.
-
Staphylococcus aureus Proteases: Orchestrators of Skin Inflammation.DNA Cell Biol. 2024 Oct;43(10):483-491. doi: 10.1089/dna.2024.0134. Epub 2024 Jul 3. DNA Cell Biol. 2024. PMID: 38957987 Review.
References
-
- Mayer-Scholl A, Averhoff P, Zychlinsky A. How do neutrophils and pathogens interact? Curr Opin Microbiol. 2004;7:62–66. - PubMed
-
- Liese JG, Jendrossek V, Jansson A, Petropoulou T, Kloos S, Gahr M, Belohradsky BH. Chronic granulomatous disease in adults. Lancet. 1996;347:220–223. - PubMed
-
- Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol. 2002;14:136–145. - PubMed
-
- Greenberg S, Chang P, Silverstein SC. Tyrosine phosphorylation of the γ subunit of Fcγ receptors, p72Syk and paxilin during Fc-receptor-mediated phagocytosis in macrophages. J Biol Chem. 1994;269:3897–902. - PubMed
-
- Mitchell MA, Huang MM, Chien P, Indik ZK, Pan XQ, Schreiber AD., Substitutions and deletions in the cytoplasmic domain of the phagocytic receptor FcγRIIA effect on receptor tyrosine phosphorylation and phagocytosis. Blood. 1994;84:1753–1759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

