Role of intestinal mucins in innate host defense mechanisms against pathogens
- PMID: 20375571
- PMCID: PMC7312850
- DOI: 10.1159/000163037
Role of intestinal mucins in innate host defense mechanisms against pathogens
Abstract
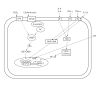
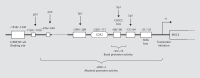
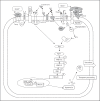
Gastrointestinal mucins produced by goblet cells comprise the main structural components of the mucus layer. Mucins play a critical role in the maintenance of mucosal homeostasis and are responsible for the differential effector and regulatory responses against a plethora of microorganisms, including commensals and pathogens. In this review, we present a comprehensive overview on mucin biology, its properties, classification and gene assembly. We also consider the structure of the mucin gene, its proteins and its role in innate host defenses. We compare the various mucin secretagogues and the differential regulatory pathways involved in mucin biosynthesis and secretion during normal and diverse pathogenic conditions. Finally, we summarize the putative uncharted aspects of mucin-derived innate host defenses, whose exploration will help drug developers to identify factors that can strengthen mucosal integrity and will facilitate basic science research into curative treatments for gastrointestinal diseases.
Copyright 2008 S. Karger AG, Basel.
Figures
References
-
- McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001;3:1–11. - PubMed
-
- Gum JR, Jr, Hicks JW, Gillespie AM, Carlson EJ, Komuves L, Karnik S, Hong JC, Epstein CJ, Kim YS. Goblet cell-specific expression mediated by the MUC2 mucin gene promoter in the intestine of transgenic mice. Am J Physiol. 1999;276:G666–G676. - PubMed
-
- Moncada DM, Chadee K. Production, structure, and function of gastrointestinal mucins. In: Blaser MJ, Infections of the Gastrointestinal Tract, editor. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. pp 57–79.
-
- Laboisse C, Jarry A, Branka JE, Merlin D, Bou-Hanna C, Vallette G. Recent aspects of the regulation of intestinal mucus secretion. Proc Nutr Soc. 1996;55:259–264. - PubMed
-
- Forstner JF, Forstner GG, Gastrointestinal mucus . Physiology of the Gastrointestinal Tract. In: Johnson LR, editor. New York: Raven Press; 1994. pp. pp 1255–1283.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

