Role of NADPH oxidase in formation and function of multinucleated giant cells
- PMID: 20375608
- PMCID: PMC2919507
- DOI: 10.1159/000228158
Role of NADPH oxidase in formation and function of multinucleated giant cells
Abstract
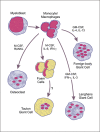
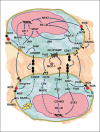
Macrophages play essential roles in a wide variety of physiological and pathological processes. One of the unique features of these phagocytic leukocytes is their ability to fuse, forming multinucleated giant cells. Multinucleated giant cells are important mediators of tissue remodeling and repair and are also responsible for removal or sequestration of foreign material, intracellular bacteria and non-phagocytosable pathogens, such as parasites and fungi. Depending on the tissue where fusion occurs and the inflammatory insult, multinucleated giant cells assume distinctly different phenotypes. Nevertheless, the ultimate outcome is the formation of large cells that can resorb bone tissue (osteoclasts) or foreign material and pathogens (giant cells) extracellularly. While progress has been made in recent years, the mechanisms and factors involved in macrophage fusion are still not fully understood. In addition to cytokines and a number of adhesion proteins and receptors, it is becoming increasingly clear that NADPH oxidase-generated reactive oxygen species (ROS) also play an important role in macrophage fusion. In this review, we provide an overview of macrophage multinucleation, with a specific focus on the role of NADPH oxidases and ROS in macrophage fusion and in the function of multinucleated giant cells. In addition, we provide an updated overview of the role of these cells in inflammation and various autoimmune diseases.
(c) 2009 S. Karger AG, Basel.
Figures

Similar articles
-
Multinucleated Giant Cells: Current Insights in Phenotype, Biological Activities, and Mechanism of Formation.Front Cell Dev Biol. 2022 Apr 11;10:873226. doi: 10.3389/fcell.2022.873226. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35478968 Free PMC article. Review.
-
Macrophage fusion and multinucleated giant cells of inflammation.Adv Exp Med Biol. 2011;713:97-111. doi: 10.1007/978-94-007-0763-4_7. Adv Exp Med Biol. 2011. PMID: 21432016 Review.
-
Molecular mediators of macrophage fusion.Trends Cell Biol. 2009 Oct;19(10):514-22. doi: 10.1016/j.tcb.2009.07.005. Epub 2009 Sep 3. Trends Cell Biol. 2009. PMID: 19733078 Review.
-
NADPH Oxidases Are Essential for Macrophage Differentiation.J Biol Chem. 2016 Sep 16;291(38):20030-41. doi: 10.1074/jbc.M116.731216. Epub 2016 Aug 3. J Biol Chem. 2016. PMID: 27489105 Free PMC article.
-
Cell fusion dynamics: mechanisms of multinucleation in osteoclasts and macrophages.Inflamm Regen. 2024 Nov 27;44(1):49. doi: 10.1186/s41232-024-00360-3. Inflamm Regen. 2024. PMID: 39605032 Free PMC article. Review.
Cited by
-
Activation of the pentose phosphate pathway in macrophages is crucial for granuloma formation in sarcoidosis.J Clin Invest. 2023 Dec 1;133(23):e171088. doi: 10.1172/JCI171088. J Clin Invest. 2023. PMID: 38038136 Free PMC article.
-
Multinucleated Giant Cells: Current Insights in Phenotype, Biological Activities, and Mechanism of Formation.Front Cell Dev Biol. 2022 Apr 11;10:873226. doi: 10.3389/fcell.2022.873226. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35478968 Free PMC article. Review.
-
Thyroglossal Duct Cyst Associated with Xanthogranulomatous Inflammation.Head Neck Pathol. 2015 Dec;9(4):530-3. doi: 10.1007/s12105-015-0628-y. Epub 2015 Apr 21. Head Neck Pathol. 2015. PMID: 25896144 Free PMC article.
-
Phenotyping of rare circulating cells in the blood of non-metastatic breast cancer patients using microfluidic Labyrinth technology.Biomicrofluidics. 2022 Dec 16;16(6):064107. doi: 10.1063/5.0129602. eCollection 2022 Dec. Biomicrofluidics. 2022. PMID: 36536791 Free PMC article.
-
Reduced Number of Adipose Lineage and Endothelial Cells in Epididymal fat in Response to Omega-3 PUFA in Mice Fed High-Fat Diet.Mar Drugs. 2018 Dec 18;16(12):515. doi: 10.3390/md16120515. Mar Drugs. 2018. PMID: 30567329 Free PMC article.
References
-
- Helming L, Gordon S. The molecular basis of macrophage fusion. Immunobiology. 2007;212:785–793. - PubMed
-
- Vignery A. Macrophage fusion: molecular mechanisms. Methods Mol Biol. 2008;475:149–161. - PubMed
-
- Langhans T. Über Riesenzellen mit wandständigen Kernen in Tuberkeln und die fibröse Form des Tuberkels. Virchows Arch Pathol Anat. 1868:382–404.
-
- Väänänen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113:377–381. - PubMed
-
- Ruibal-Ares B, Riera NE, de Bracco MM. Macrophages, multinucleated giant cells, and apoptosis in HIV+ patients and normal blood donors. Clin Immunol Immunopathol. 1997;82:102–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

