Synthesis and antimicrobial evaluation of 5-iodopyrimidine analogs
- PMID: 20376222
- PMCID: PMC2846474
- DOI: 10.4103/0250-474X.59551
Synthesis and antimicrobial evaluation of 5-iodopyrimidine analogs
Abstract
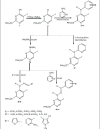
4-Substituted-5-iodo-2-benzylthiopyrimidines were prepared efficiently in three steps. 2-Benzylthiopyrimidine on iodination in presence of base gave 5-iodo-2-benzylthiopyrimidine (1), which on chlorination with excess of POCl(3) furnished 4-chloro-5-iodo-2-benzylthiopyrimidine (2). Reaction of 2 with substituted aromatic amines, 2-aminopyridine and hydrazine hydrate yielded 4-amino-5-iodo-2-benzylthiopyrimidines 3(a-e), (3f) and (3g) respectively. Further, 4-hydrazino-5-iodo-2-benzylthiopyrimidine on condensation with substituted aromatic and heterocyclic aldehydes afforded the corresponding schiff bases 4(a-h). The structure of synthesized compounds have been established by spectral studies and elemental analysis. Synthesized compounds have been screened for antimicrobial activity. Compound 3f exhibited good antifungal activity against A. niger. The compounds 4a, 4c, 4d, 4g and 4h exhibited good antibacterial activity.
Keywords: 5-Substituted pyrimidine; Antibacterial; antifungal; schiff bases.
Figures
Similar articles
-
Synthesis of novel 1,3,4-oxadiazole derivatives as potential antimicrobial agents.Acta Pol Pharm. 2010 May-Jun;67(3):247-53. Acta Pol Pharm. 2010. PMID: 20524426
-
Synthesis of some new 4-styryltetrazolo[1,5-a]quinoxaline and 1-substituted-4-styryl[1,2,4]triazolo[4,3-a]quinoxaline derivatives as potent anticonvulsants.Eur J Med Chem. 2009 Mar;44(3):1135-43. doi: 10.1016/j.ejmech.2008.06.006. Epub 2008 Jun 20. Eur J Med Chem. 2009. PMID: 18672315
-
Facile Synthesis, Characterization, and Antimicrobial Evaluation of Novel Heterocycles, Schiff Bases, and N-Nucleosides Bearing Phthalazine Moiety.Chem Pharm Bull (Tokyo). 2016;64(5):439-50. doi: 10.1248/cpb.c15-01005. Chem Pharm Bull (Tokyo). 2016. PMID: 27150476
-
Synthesis and antimicrobial activity of 7-(2-substituted phenylthiazolidinyl)-benzopyran-2-one derivatives.Eur J Med Chem. 2010 Jan;45(1):85-9. doi: 10.1016/j.ejmech.2009.09.028. Epub 2009 Sep 23. Eur J Med Chem. 2010. PMID: 19837487
-
Synthesis and biological activity of N-substituted-3-chloro-2-azetidinones.Molecules. 2007 Nov 12;12(11):2467-77. doi: 10.3390/12112467. Molecules. 2007. PMID: 18065951 Free PMC article.
Cited by
-
Investigation of embelin synthetic hybrids as potential COVID-19 and COX inhibitors: Synthesis, spectral analysis, DFT calculations and molecular docking studies.J Mol Struct. 2023 Feb 5;1273:134356. doi: 10.1016/j.molstruc.2022.134356. Epub 2022 Oct 17. J Mol Struct. 2023. PMID: 36277303 Free PMC article.
-
Recent medicinal approaches of novel pyrimidine analogs: A review.Heliyon. 2023 Jun 2;9(6):e16773. doi: 10.1016/j.heliyon.2023.e16773. eCollection 2023 Jun. Heliyon. 2023. PMID: 37346348 Free PMC article. Review.
-
Therapeutic potential of pyrrole and pyrrolidine analogs: an update.Mol Divers. 2022 Oct;26(5):2915-2937. doi: 10.1007/s11030-022-10387-8. Epub 2022 Jan 25. Mol Divers. 2022. PMID: 35079946 Free PMC article. Review.
References
-
- Jain KS, Chitre TS, Maniyar PB, Kathiravan MK, Bindre VS, Veer VS, et al. Biological and medicinal significance of pyrimidine. Current Sci. 2006;90:793–803.
-
- Mitsuya H, Weinhold KJ, Furman PA, St.Clair MH, Nusinoff-Lehman S, Gallo RC, et al. 3'-Azido-3'-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci USA. 1985;82:7096–100. - PMC - PubMed
-
- Lin TS, Guo JY, Schinazi RF, Chu CK, Xiang JN, Prusoff WH. Synthesis and antiviral activity of various 3-azido analogues of pyrimidine deoxyribonucleosides against human immunodeficiency virus (HIV-1, HTLV-III/LAV) J Med Chem. 1988;31:336–40. - PubMed
-
- Choo H, Chong Y, Choi Y, Mathew J, Schinazi RF, Chu CK. Synthesis, anti-HIV activity and molecular mechanism of drug resistance of L-2'.3'-didehydro-2',3'-dideoxy-2'-fluoro-4'-thionucleosides. J Med Chem. 2003;46:389–98. - PubMed
LinkOut - more resources
Full Text Sources