Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease
- PMID: 20376313
- PMCID: PMC2848616
- DOI: 10.1371/journal.pone.0009979
Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease
Erratum in
- PLoS One. 2011;6(11). doi:10.1371/annotation/05c1b976-7eab-4154-808d-0526e604b8eb
Abstract
Background: Reduced TOR signaling has been shown to significantly increase lifespan in a variety of organisms [1], [2], [3], [4]. It was recently demonstrated that long-term treatment with rapamycin, an inhibitor of the mTOR pathway[5], or ablation of the mTOR target p70S6K[6] extends lifespan in mice, possibly by delaying aging. Whether inhibition of the mTOR pathway would delay or prevent age-associated disease such as AD remained to be determined.
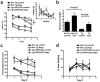
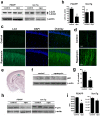
Methodology/principal findings: We used rapamycin administration and behavioral tools in a mouse model of AD as well as standard biochemical and immunohistochemical measures in brain tissue to provide answers for this question. Here we show that long-term inhibition of mTOR by rapamycin prevented AD-like cognitive deficits and lowered levels of Abeta(42), a major toxic species in AD[7], in the PDAPP transgenic mouse model. These data indicate that inhibition of the mTOR pathway can reduce Abeta(42) levels in vivo and block or delay AD in mice. As expected from the inhibition of mTOR, autophagy was increased in neurons of rapamycin-treated transgenic, but not in non-transgenic, PDAPP mice, suggesting that the reduction in Abeta and the improvement in cognitive function are due in part to increased autophagy, possibly as a response to high levels of Abeta.
Conclusions/significance: Our data suggest that inhibition of mTOR by rapamycin, an intervention that extends lifespan in mice, can slow or block AD progression in a transgenic mouse model of the disease. Rapamycin, already used in clinical settings, may be a potentially effective therapeutic agent for the treatment of AD.
Conflict of interest statement
Figures
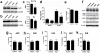
References
-
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. - PubMed
-
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. - PubMed
-
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, et al. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

