Updating systematic reviews: an international survey
- PMID: 20376338
- PMCID: PMC2848577
- DOI: 10.1371/journal.pone.0009914
Updating systematic reviews: an international survey
Abstract
Background: Systematic reviews (SRs) should be up to date to maintain their importance in informing healthcare policy and practice. However, little guidance is available about when and how to update SRs. Moreover, the updating policies and practices of organizations that commission or produce SRs are unclear.
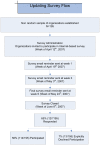
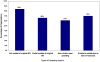
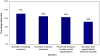
Methodology/principal findings: The objective was to describe the updating practices and policies of agencies that sponsor or conduct SRs. An Internet-based survey was administered to a purposive non-random sample of 195 healthcare organizations within the international SR community. Survey results were analyzed using descriptive statistics. The completed response rate was 58% (n = 114) from across 26 countries with 70% (75/107) of participants identified as producers of SRs. Among responders, 79% (84/107) characterized the importance of updating as high or very-high and 57% (60/106) of organizations reported to have a formal policy for updating. However, only 29% (35/106) of organizations made reference to a written policy document. Several groups (62/105; 59%) reported updating practices as irregular, and over half (53/103) of organizational respondents estimated that more than 50% of their respective SRs were likely out of date. Authors of the original SR (42/106; 40%) were most often deemed responsible for ensuring SRs were current. Barriers to updating included resource constraints, reviewer motivation, lack of academic credit, and limited publishing formats. Most respondents (70/100; 70%) indicated that they supported centralization of updating efforts across institutions or agencies. Furthermore, 84% (83/99) of respondents indicated they favoured the development of a central registry of SRs, analogous to efforts within the clinical trials community.
Conclusions/significance: Most organizations that sponsor and/or carry out SRs consider updating important. Despite this recognition, updating practices are not regular, and many organizations lack a formal written policy for updating SRs. This research marks the first baseline data available on updating from an organizational perspective.
Conflict of interest statement
Figures
References
-
- Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, et al. How quickly do systematic reviews go out of date. A survival analysis. Ann Intern Med. 2007;147:224–233. - PubMed
-
- Moher D, Tsertsvadze A. Systematic reviews: when is an update an update? Lancet. 2006;367(9514):881–883. - PubMed
-
- Higgins JPT, Green S (eds)., editors. 2008. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org.
-
- Dillon A. (2005) Updating Guidelines and Correcting Errors. Guideline Development Methods. Wetherby, Yorkshire: National Institute for Health and Clinical Excellence. Available from www.nice.org.uk/pdf/GDM_Chapter15.pdf.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials