HSPG-binding peptide corresponding to the exon 6a-encoded domain of VEGF inhibits tumor growth by blocking angiogenesis in murine model
- PMID: 20376344
- PMCID: PMC2848586
- DOI: 10.1371/journal.pone.0009945
HSPG-binding peptide corresponding to the exon 6a-encoded domain of VEGF inhibits tumor growth by blocking angiogenesis in murine model
Abstract
Vascular endothelial growth factor VEGF(165) is a critical element for development of the vascular system in physiological and pathological angiogenesis. VEGF isoforms have different affinities for heparan sulphate proteoglycan (HSPG) as well as for VEGF receptors; HSPGs are important regulators in vascular development. Therefore, inhibition of interactions between VEGF and HSPGs may prevent angiogenesis. Here, we demonstrate that an HSPG-binding synthetic peptide, corresponding to exon 6a-encoded domain of VEGF gene, has anti-angiogenic property. This 20 amino acids synthetic peptide prevents VEGF(165) binding to several different cell types, mouse embryonic sections and inhibits endothelial cell migration, despite its absence in VEGF(165) sequence. Our in vivo anti-tumor studies show that the peptide inhibits tumor growth in both mouse Lewis-Lung Carcinoma and human Liposarcoma tumor-bearing animal models. This is the first evidence that a synthetic VEGF fragment corresponding to exon 6a has functional antagonism both in vitro and in vivo. We conclude that the above HPSG binding peptide (6a-P) is a potent inhibitor of angiogenesis-dependent diseases.
Conflict of interest statement
Figures
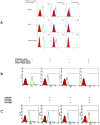
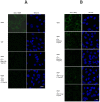
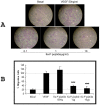
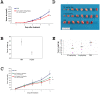
References
-
- Folkman J. Angiogenesis. Annu Rev Med; 2006;57:1–18. - PubMed
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. - PubMed
-
- Robinson JC, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Scien. 2001;114:853–65. - PubMed
-
- Hervě MA, Buteau-Lozanoa H, Mourahb S, Calvob F, Perrot-Applanat M. VEGF189 stimulates endothelial cells proliferation and migration in vitro and up-regulates the expression of Flk-1/KDR mRNA. Exp Cell Res. 2005;309:24–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

