Quantification of rapid Myosin regulatory light chain phosphorylation using high-throughput in-cell Western assays: comparison to Western immunoblots
- PMID: 20376358
- PMCID: PMC2848601
- DOI: 10.1371/journal.pone.0009965
Quantification of rapid Myosin regulatory light chain phosphorylation using high-throughput in-cell Western assays: comparison to Western immunoblots
Abstract
Background: Quantification of phospho-proteins (PPs) is crucial when studying cellular signaling pathways. Western immunoblotting (WB) is commonly used for the measurement of relative levels of signaling intermediates in experimental samples. However, WB is in general a labour-intensive and low-throughput technique. Because of variability in protein yield and phospho-signal preservation during protein harvesting, and potential loss of antigen during protein transfer, WB provides only semi-quantitative data. By comparison, the "in-cell western" (ICW) technique has high-throughput capacity and requires less extensive sample preparation. Thus, we compared the ICW technique to WB for measuring phosphorylated myosin regulatory light chain (PMLC(20)) in primary cultures of uterine myocytes to assess their relative specificity, sensitivity, precision, and quantification of biologically relevant responses.
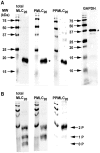
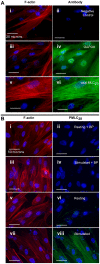
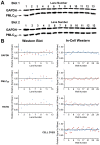
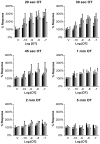
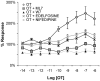
Methodology/principal findings: ICWs are cell-based microplate assays for quantification of protein targets in their cellular context. ICWs utilize a two-channel infrared (IR) scanner (Odyssey(R)) to quantify signals arising from near-infrared (NIR) fluorophores conjugated to secondary antibodies. One channel is dedicated to measuring the protein of interest and the second is used for data normalization of the signal in each well of the microplate. Using uterine myocytes, we assessed oxytocin (OT)-stimulated MLC(20) phosphorylation measured by ICW and WB, both using NIR fluorescence. ICW and WB data were comparable regarding signal linearity, signal specificity, and time course of phosphorylation response to OT.
Conclusion/significance: ICW and WB yield comparable biological data. The advantages of ICW over WB are its high-throughput capacity, improved precision, and reduced sample preparation requirements. ICW might provide better sensitivity and precision with low-quantity samples or for protocols requiring large numbers of samples. These features make the ICW technique an excellent tool for the study of phosphorylation endpoints. However, the drawbacks of ICW include the need for a cell culture format and the lack of utility where protein purification, concentration or stoichiometric analyses are required.
Conflict of interest statement
Figures



References
-
- Weldon S, Ambroz K, Schutz-Geschwender A, Olive DM. Near-infrared fluorescence detection permits accurate imaging of loading controls for Western blot analysis. Anal Biochem. 2008;375:156–158. - PubMed
-
- Jungblut P, Eckerskorn C, Lottspeich F, Klose J. Blotting efficiency investigated by using two-dimensional electrophoresis, hydrophobic membranes and proteins from different sources. Electrophoresis. 1990;11:581–588. - PubMed
-
- Tovey ER, Baldo BA. Comparison of semi-dry and conventional tank-buffer electrotransfer of proteins from polyacrylamide gels to nitrocellulose membranes. Electrophoresis. 1987;8:384–387.
-
- Selkirk JV, Nottebaum LM, Ford IC, Santos M, Malany S, et al. A novel cell-based assay for G-protein-coupled receptor-mediated cyclic adenosine monophosphate response element binding protein phosphorylation. J Biomol Screen. 2006;11:351–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

