Diagnostic approach for the differentiation of the pandemic influenza A(H1N1)v virus from recent human influenza viruses by real-time PCR
- PMID: 20376359
- PMCID: PMC2848602
- DOI: 10.1371/journal.pone.0009966
Diagnostic approach for the differentiation of the pandemic influenza A(H1N1)v virus from recent human influenza viruses by real-time PCR
Abstract
Background: The current spread of pandemic influenza A(H1N1)v virus necessitates an intensified surveillance of influenza virus infections worldwide. So far, in many laboratories routine diagnostics were limited to generic influenza virus detection only. To provide interested laboratories with real-time PCR assays for type and subtype identification, we present a bundle of PCR assays with which any human influenza A and B virus can be easily identified, including assays for the detection of the pandemic A(H1N1)v virus.
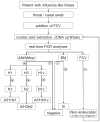
Principal findings: The assays show optimal performance characteristics in their validation on plasmids containing the respective assay target sequences. All assays have furthermore been applied to several thousand clinical samples since 2007 (assays for seasonal influenza) and April 2009 (pandemic influenza assays), respectively, and showed excellent results also on clinical material.
Conclusions: We consider the presented assays to be well suited for the detection and subtyping of circulating influenza viruses.
Conflict of interest statement
Figures
Similar articles
-
Design and validation of real-time reverse transcription-PCR assays for detection of pandemic (H1N1) 2009 virus.J Clin Microbiol. 2009 Nov;47(11):3454-60. doi: 10.1128/JCM.01103-09. Epub 2009 Sep 2. J Clin Microbiol. 2009. PMID: 19726603 Free PMC article.
-
Comparison of a singleplex real-time RT-PCR assay and multiplex respiratory viral panel assay for detection of influenza "A" in respiratory specimens.Influenza Other Respir Viruses. 2011 Mar;5(2):99-103. doi: 10.1111/j.1750-2659.2010.00170.x. Influenza Other Respir Viruses. 2011. PMID: 21244644 Free PMC article.
-
Development and comparison of molecular assays for the rapid detection of the pandemic influenza A (H1N1) 2009 virus.J Med Virol. 2010 Apr;82(4):675-83. doi: 10.1002/jmv.21725. J Med Virol. 2010. PMID: 20166184
-
Update on influenza diagnostics: lessons from the novel H1N1 influenza A pandemic.Clin Microbiol Rev. 2012 Apr;25(2):344-61. doi: 10.1128/CMR.05016-11. Clin Microbiol Rev. 2012. PMID: 22491775 Free PMC article. Review.
-
[The pandemic influenza virus H1N1/2009: a review of the molecular biology, phylogeny, history of reassortments, and parameters of host switching].Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2010 Dec;53(12):1231-7. doi: 10.1007/s00103-010-1166-0. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2010. PMID: 21161472 Review. German.
Cited by
-
Comparison of shedding characteristics of seasonal influenza virus (sub)types and influenza A(H1N1)pdm09; Germany, 2007-2011.PLoS One. 2012;7(12):e51653. doi: 10.1371/journal.pone.0051653. Epub 2012 Dec 11. PLoS One. 2012. PMID: 23240050 Free PMC article.
-
Predicting AH1N1 2009 influenza epidemic in Southeast Europe.Croat Med J. 2011 Apr 15;52(2):115-25. doi: 10.3325/cmj.2011.52.115. Croat Med J. 2011. PMID: 21495193 Free PMC article.
-
Dual Detection of Hemagglutinin Proteins of H5N1 and H1N1 Influenza Viruses Based on FRET Combined With DNase I.Front Microbiol. 2022 Jun 30;13:934475. doi: 10.3389/fmicb.2022.934475. eCollection 2022. Front Microbiol. 2022. PMID: 35847124 Free PMC article.
-
Protocol for metagenomic virus detection in clinical specimens.Emerg Infect Dis. 2015 Jan;21(1):48-57. doi: 10.3201/eid2101.140766. Emerg Infect Dis. 2015. PMID: 25532973 Free PMC article.
-
The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009-2011.BMC Infect Dis. 2012 Jan 26;12:26. doi: 10.1186/1471-2334-12-26. BMC Infect Dis. 2012. PMID: 22280120 Free PMC article. Clinical Trial.
References
-
- Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Fifth ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1691–1740.
-
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. - PubMed
-
- World Health Organisation website. Influenza (Seasonal) Fact Sheet. 2010. Available: http://www.who.int/mediacentre/factsheets/fs211/en/
-
- Hurt AC, Ernest J, Deng YM, Iannello P, Besselaar TG, et al. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antiviral Res. 2009;83:90–93. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

