Characterizing the anti-tumor function of adoptively transferred NK cells in vivo
- PMID: 20376439
- PMCID: PMC11030891
- DOI: 10.1007/s00262-010-0848-7
Characterizing the anti-tumor function of adoptively transferred NK cells in vivo
Abstract
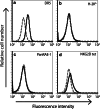
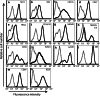
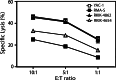
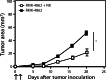
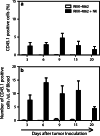
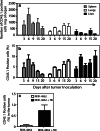
Natural killer (NK) cells represent a promising cell type to utilize for effective adoptive immunotherapy. However, little is known about the important cytolytic molecules and signaling pathways used by NK cells in the adoptive transfer setting. To address this issue, we developed a novel mouse model to investigate the trafficking and mechanism of action of these cells. We demonstrate that methylcholanthrene-induced RKIK sarcoma cells were susceptible to NK cell-mediated lysis in vitro and in vivo following adoptive transfer of NK cells in C57BL/6 RAG-2(-/-)gammac(-/-) mice. Cytotoxic molecules perforin, granzymes B and M as well as the death ligand TRAIL and pro-inflammatory cytokine IFN-gamma were found to be important in the anti-tumor effect mediated by adoptively transferred NK cells. Importantly, we demonstrate that adoptively transferred NK cells could traffic to the tumor site and persisted in vivo which correlated with the anti-tumor effect observed. Overall, the results of this study have important implications for enhancing NK cell-based immunotherapies.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

