Three-dimensional finite element modeling of pericellular matrix and cell mechanics in the nucleus pulposus of the intervertebral disk based on in situ morphology
- PMID: 20376522
- PMCID: PMC2970666
- DOI: 10.1007/s10237-010-0214-x
Three-dimensional finite element modeling of pericellular matrix and cell mechanics in the nucleus pulposus of the intervertebral disk based on in situ morphology
Abstract
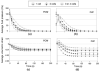
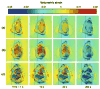
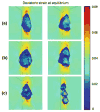
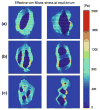
Nucleus pulposus (NP) cells of the intervertebral disk (IVD) have unique morphological characteristics and biologic responses to mechanical stimuli that may regulate maintenance and health of the IVD. NP cells reside as single cell, paired or multiple cells in a contiguous pericellular matrix (PCM), whose structure and properties may significantly influence cell and extracellular matrix mechanics. In this study, a computational model was developed to predict the stress-strain, fluid pressure and flow fields for cells and their surrounding PCM in the NP using three-dimensional (3D) finite element models based on the in situ morphology of cell-PCM regions of the mature rat NP, measured using confocal microscopy. Three-dimensional geometries of the extracellular matrix and representative cell-matrix units were used to construct 3D finite element models of the structures as isotropic and biphasic materials. In response to compressive strain of the extracellular matrix, NP cells and PCM regions were predicted to experience volumetric strains that were 1.9-3.7 and 1.4-2.1 times greater than the extracellular matrix, respectively. Volumetric and deviatoric strain concentrations were generally found at the cell/PCM interface, while von Mises stress concentrations were associated with the PCM/extracellular matrix interface. Cell-matrix units containing greater cell numbers were associated with higher peak cell strains and lower rates of fluid pressurization upon loading. These studies provide new model predictions for micromechanics of NP cells that can contribute to an understanding of mechanotransduction in the IVD and its changes with aging and degeneration.
Figures


References
-
- Alexopoulos LG, Haider MA, Vail TP, Guilak F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J Biomech Eng. 2003;125(3):323–333. - PubMed
-
- Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005a;1(3):317–325. - PubMed
-
- Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomech. 2005b;38(3):509–517. - PubMed
-
- Baer AE, Laursen TA, Guilak F, Setton LA. The micromechanical environment of intervertebral disc cells determined by a finite deformation, anisotropic, and biphasic finite element model. J Biomech Eng. 2003;125(1):1–11. - PubMed
-
- Baer AE, Setton LA. The micromechanical environment of intervertebral disc cells: effect of matrix anisotropy and cell geometry predicted by a linear model. J Biomech Eng. 2000;122(3):245–251. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

