Viral shedding and clinical illness in naturally acquired influenza virus infections
- PMID: 20377412
- PMCID: PMC3060408
- DOI: 10.1086/652241
Viral shedding and clinical illness in naturally acquired influenza virus infections
Abstract
Background: Volunteer challenge studies have provided detailed data on viral shedding from the respiratory tract before and through the course of experimental influenza virus infection. There are no comparable quantitative data to our knowledge on naturally acquired infections.
Methods: In a community-based study in Hong Kong in 2008, we followed up initially healthy individuals to quantify trends in viral shedding on the basis of cultures and reverse-transcription polymerase chain reaction (RT-PCR) through the course of illness associated with seasonal influenza A and B virus infection.
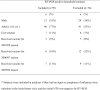
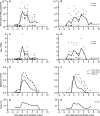
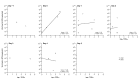
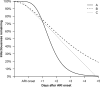
Results: Trends in symptom scores more closely matched changes in molecular viral loads measured with RT-PCR for influenza A than for influenza B. For influenza A virus infections, the replicating viral loads determined with cultures decreased to undetectable levels earlier after illness onset than did molecular viral loads. Most viral shedding occurred during the first 2-3 days after illness onset, and we estimated that 1%-8% of infectiousness occurs prior to illness onset. Only 14% of infections with detectable shedding at RT-PCR were asymptomatic, and viral shedding was low in these cases.
Conclusions: Our results suggest that "silent spreaders" (ie, individuals who are infectious while asymptomatic or presymptomatic) may be less important in the spread of influenza epidemics than previously thought.
Conflict of interest statement
Potential conflicts of interest: none reported.
Figures



References
-
- Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167:775–785. - PubMed
-
- Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151:437–446. - PubMed
-
- Cheng CK, Cowling BJ, Chan KH, et al. Factors affecting QuickVue Influenza A + B rapid test performance in the community setting. Diagn Microbiol Infect Dis. 2009;65:35–41. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

