Depo-Provera does not alter disease progression in SIVmac-infected female Chinese rhesus macaques
- PMID: 20377424
- PMCID: PMC2864058
- DOI: 10.1089/aid.2009.0185
Depo-Provera does not alter disease progression in SIVmac-infected female Chinese rhesus macaques
Abstract
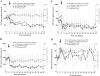
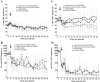
Depo-Provera (medroxyprogesterone acetate), a long-acting derivative of progesterone, is utilized during many nonhuman primate microbicide studies to facilitate simian immunodeficiency virus (SIV) infection by thinning the vaginal epithelium. To date, the systemic effects of this steroid hormone in regard to SIV/HIV pathogenesis are not well understood, but an increase in infection rates and lymphoproliferation following progesterone application has been reported. Therefore, a proactive study using 20 Chinese rhesus macaques was designed to investigate the effect of a single Depo-Provera injection on SIV disease progression. Group 1 (n = 10) was treated with 30 mg Depo-Provera intramuscularly 30 days prior to intravenous challenge with 50 TCID(50) SIVmac251, while Group 2 (n = 10) remained untreated, but received the same amount of SIV. Blood samples were taken at predetermined intervals to measure RNA viral loads, CD4(+), CD8(+), and CD20(+) lymphocyte counts and percentages and absolute numbers of naive and memory T lymphocytes. Upon statistical endpoint data analysis, none of the parameters measured were shown to be significantly different between the groups. One animal in the Depo-Provera-treated group and two macaques in the control group were euthanized prior to study end due to the development of clinical signs (in weeks 43 and 51, respectively). All other animals were euthanized between weeks 68 and 71 post-SIV infection. Histopathological evaluations revealed that 5 of 10 animals in each group had developed simian AIDS (SAIDS). In summary, this prospective study demonstrated that a single injection of 30 mg Depo-Provera did not have a significant influence on SIV disease progression.
Figures
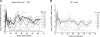
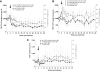

Similar articles
-
Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of depo-provera before intravaginal challenge with simian immunodeficiency virus mac239.J Infect Dis. 2004 Nov 1;190(9):1697-705. doi: 10.1086/424600. Epub 2004 Sep 24. J Infect Dis. 2004. PMID: 15478078 Free PMC article.
-
Depo-provera treatment does not abrogate protection from intravenous SIV challenge in female macaques immunized with an attenuated AIDS virus.PLoS One. 2010 Mar 23;5(3):e9814. doi: 10.1371/journal.pone.0009814. PLoS One. 2010. PMID: 20352116 Free PMC article.
-
Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques.Virology. 2006 Aug 15;352(1):169-77. doi: 10.1016/j.virol.2006.04.004. Epub 2006 May 30. Virology. 2006. PMID: 16730772
-
SIV infection of rhesus macaques of Chinese origin: a suitable model for HIV infection in humans.Retrovirology. 2013 Aug 15;10:89. doi: 10.1186/1742-4690-10-89. Retrovirology. 2013. PMID: 23947613 Free PMC article. Review.
-
Cannabinoid administration attenuates the progression of simian immunodeficiency virus.AIDS Res Hum Retroviruses. 2011 Jun;27(6):585-92. doi: 10.1089/aid.2010.0218. Epub 2010 Nov 23. AIDS Res Hum Retroviruses. 2011. PMID: 20874519 Free PMC article. Review.
Cited by
-
Animal models for microbicide studies.Curr HIV Res. 2012 Jan 1;10(1):79-87. doi: 10.2174/157016212799304715. Curr HIV Res. 2012. PMID: 22264049 Free PMC article. Review.
-
Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication.J Clin Invest. 2014 Jun;124(6):2802-6. doi: 10.1172/JCI75090. Epub 2014 May 16. J Clin Invest. 2014. PMID: 24837437 Free PMC article.
-
Macaque studies of vaccine and microbicide combinations for preventing HIV-1 sexual transmission.Proc Natl Acad Sci U S A. 2012 May 29;109(22):8694-8. doi: 10.1073/pnas.1203183109. Epub 2012 May 14. Proc Natl Acad Sci U S A. 2012. PMID: 22586094 Free PMC article.
-
Nonhuman primate models for the evaluation of HIV-1 preventive vaccine strategies: model parameter considerations and consequences.Curr Opin HIV AIDS. 2016 Nov;11(6):546-554. doi: 10.1097/COH.0000000000000311. Curr Opin HIV AIDS. 2016. PMID: 27559710 Free PMC article. Review.
-
Plasma concentration of injectable contraceptive correlates with reduced cervicovaginal growth factor expression in South African women.Mucosal Immunol. 2020 May;13(3):449-459. doi: 10.1038/s41385-019-0249-y. Epub 2020 Jan 2. Mucosal Immunol. 2020. PMID: 31896762 Free PMC article.
References
-
- Fauci AS. 25 years of HIV/AIDS science: Reaching the poor with research advances. Cell. 2007;131:429–432. - PubMed
-
- Flores J. Seeking new pathways for HIV vaccine discovery. Future Microbiol. 2009;4:1–7. - PubMed
-
- Turpin JA. Schito ML. Jenkins LM. Inman JK. Appella E. Topical microbicides: A promising approach for controlling the AIDS pandemic via retroviral zinc finger inhibitors. Adv Pharmacol. 2008;56:229–256. - PubMed
-
- Klasse PJ. Shattock R. Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–471. - PubMed
-
- McGowan I. Microbicides: A new frontier in HIV prevention. Biologicals. 2006;34:241–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
