Nutraceutical augmentation of circulating endothelial progenitor cells and hematopoietic stem cells in human subjects
- PMID: 20377846
- PMCID: PMC2862021
- DOI: 10.1186/1479-5876-8-34
Nutraceutical augmentation of circulating endothelial progenitor cells and hematopoietic stem cells in human subjects
Abstract
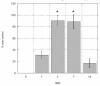
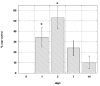
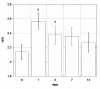
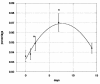
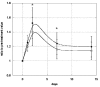
The medical significance of circulating endothelial or hematopoietic progenitors is becoming increasing recognized. While therapeutic augmentation of circulating progenitor cells using G-CSF has resulted in promising preclinical and early clinical data for several degenerative conditions, this approach is limited by cost and inability to perform chronic administration. Stem-Kine is a food supplement that was previously reported to augment circulating EPC in a pilot study. Here we report a trial in 18 healthy volunteers administered Stem-Kine twice daily for a 2 week period. Significant increases in circulating CD133 and CD34 cells were observed at days 1, 2, 7, and 14 subsequent to initiation of administration, which correlated with increased hematopoietic progenitors as detected by the HALO assay. Augmentation of EPC numbers in circulation was detected by KDR-1/CD34 staining and colony forming assays. These data suggest Stem-Kine supplementation may be useful as a stimulator of reparative processes associated with mobilization of hematopoietic and endothelial progenitors.
Figures

Similar articles
-
Different subpopulations of endothelial progenitor cells and circulating apoptotic progenitor cells in patients with vascular disease and diabetes.Int J Cardiol. 2010 Sep 3;143(3):368-72. doi: 10.1016/j.ijcard.2009.03.075. Epub 2009 Apr 26. Int J Cardiol. 2010. PMID: 19398138
-
Absence of a relationship between immunophenotypic and colony enumeration analysis of endothelial progenitor cells in clinical haematopoietic cell sources.J Transl Med. 2007 Jul 18;5:37. doi: 10.1186/1479-5876-5-37. J Transl Med. 2007. PMID: 17640360 Free PMC article.
-
Evaluation of mobilized peripheral blood CD34(+) cells from patients with severe coronary artery disease as a source of endothelial progenitor cells.Cytotherapy. 2010 Apr;12(2):178-89. doi: 10.3109/14653240903493409. Cytotherapy. 2010. PMID: 20078384 Free PMC article.
-
Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease.Arterioscler Thromb Vasc Biol. 2005 Feb;25(2):296-301. doi: 10.1161/01.ATV.0000151690.43777.e4. Epub 2004 Nov 29. Arterioscler Thromb Vasc Biol. 2005. PMID: 15569821 Clinical Trial.
-
Clonogenic assay of endothelial progenitor cells.Trends Cardiovasc Med. 2013 May;23(4):99-103. doi: 10.1016/j.tcm.2012.09.007. Epub 2013 Feb 1. Trends Cardiovasc Med. 2013. PMID: 23375595 Review.
Cited by
-
Could metabolic syndrome, lipodystrophy, and aging be mesenchymal stem cell exhaustion syndromes?Stem Cells Int. 2011;2011:943216. doi: 10.4061/2011/943216. Epub 2011 Jun 13. Stem Cells Int. 2011. PMID: 21716667 Free PMC article.
-
Upregulation of circulating cancer stem cell marker, DCLK1 but not Lgr5, in chemoradiotherapy-treated colorectal cancer patients.Tumour Biol. 2015 Jun;36(6):4801-10. doi: 10.1007/s13277-015-3132-9. Epub 2015 Jan 29. Tumour Biol. 2015. PMID: 25631749
-
Markers and Biomarkers of Endothelium: When Something Is Rotten in the State.Oxid Med Cell Longev. 2017;2017:9759735. doi: 10.1155/2017/9759735. Epub 2017 Nov 23. Oxid Med Cell Longev. 2017. PMID: 29333215 Free PMC article. Review.
-
Effect of Infla-Kine supplementation on the gene expression of inflammatory markers in peripheral mononuclear cells and on C-reactive protein in blood.J Transl Med. 2017 Oct 20;15(1):213. doi: 10.1186/s12967-017-1315-4. J Transl Med. 2017. PMID: 29058588 Free PMC article. Clinical Trial.
-
Hematopoietic Stem Cell: Regulation and Nutritional Intervention.Nutrients. 2023 Jun 1;15(11):2605. doi: 10.3390/nu15112605. Nutrients. 2023. PMID: 37299568 Free PMC article. Review.
References
-
- Fischer-Rasokat U, Assmus B, Seeger FH. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circ Heart Fail. 2009;2(5):417–23. doi: 10.1161/CIRCHEARTFAILURE.109.855023. - DOI - PubMed
-
- Suarez-Monteagudo C, Hernandez-Ramirez P, Alvarez-Gonzalez L. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27(3):151–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

