The transition from noncoded to coded protein synthesis: did coding mRNAs arise from stability-enhancing binding partners to tRNA?
- PMID: 20377916
- PMCID: PMC2859854
- DOI: 10.1186/1745-6150-5-16
The transition from noncoded to coded protein synthesis: did coding mRNAs arise from stability-enhancing binding partners to tRNA?
Abstract
Background: Understanding the origin of protein synthesis has been notoriously difficult. We have taken as a starting premise Wolf and Koonin's view that "evolution of the translation system is envisaged to occur in a compartmentalized ensemble of replicating, co-selected RNA segments, i.e., in an RNA world containing ribozymes with versatile activities".
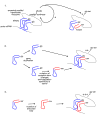
Presentation of the hypothesis: We propose that coded protein synthesis arose from a noncoded process in an RNA world as a natural consequence of the accumulation of a range of early tRNAs and their serendipitous RNA binding partners. We propose that, initially, RNA molecules with 3' CCA termini that could be aminoacylated by ribozymes, together with an ancestral peptidyl transferase ribozyme, produced small peptides with random or repetitive sequences. Our concept is that the first tRNA arose in this context from the ligation of two RNA hairpins and could be similarly aminoacylated at its 3' end to become a substrate for peptidyl transfer catalyzed by the ancestral ribozyme. Within this RNA world we hypothesize that proto-mRNAs appeared first simply as serendipitous binding partners, forming complementary base pair interactions with the anticodon loops of tRNA pairs. Initially this may have enhanced stability of the paired tRNA molecules so they were held together in close proximity, better positioning the 3' CCA termini for peptidyl transfer and enhancing the rate of peptide synthesis. If there were a selective advantage for the ensemble through the peptide products synthesized, it would provide a natural pathway for the evolution of a coding system with the expansion of a cohort of different tRNAs and their binding partners. The whole process could have occurred quite unremarkably for such a profound acquisition.
Testing the hypothesis: It should be possible to test the different parts of our model using the isolated contemporary 50S ribosomal subunit initially, and then with RNAs transcribed in vitro together with a minimal set of ribosomal proteins that are required today to support protein synthesis.
Implications of the hypothesis: This model proposes that genetic coding arose de novo from complementary base pair interactions between tRNAs and single-stranded RNAs present in the immediate environment.
Reviewers: This article was reviewed by Eugene Koonin, Rob Knight and Berthold Kastner (nominated by Laura Landweber).
Figures




References
-
- Weiss R, Cherry J. In: The RNA World. Gesterland RF, Atkins JF, editor. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1993. Speculations on the origin of ribosomal translocation; pp. 71–89.
-
- Penny D. An interpretive review of the origin of life research. Biol Philos. 2005;20:633–671. doi: 10.1007/s10539-004-7342-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

