Chronic stress causes amygdala hyperexcitability in rodents
- PMID: 20378100
- PMCID: PMC2882519
- DOI: 10.1016/j.biopsych.2010.02.008
Chronic stress causes amygdala hyperexcitability in rodents
Abstract
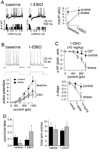
Background: Chronic stress is a major health concern, often leading to depression, anxiety, or when severe enough, posttraumatic stress disorder. While many studies demonstrate that the amygdala is hyperresponsive in patients with these disorders, the cellular neurophysiological effects of chronic stress on the systems that underlie psychiatric disorders, such as the amygdala, are relatively unknown.
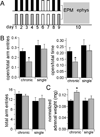
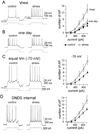
Methods: In this study, we examined the effects of chronic stress on the activity and excitability of amygdala neurons in vivo in rats. We used in vivo intracellular recordings from single neurons of the lateral amygdala (LAT) to measure neuronal properties and determine the cellular mechanism for the effects of chronic stress on LAT neurons.
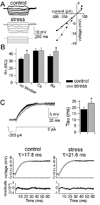
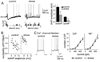
Results: We found a mechanism for the effects of chronic stress on amygdala activity, specifically that chronic stress increased excitability of LAT pyramidal neurons recorded in vivo. This hyperexcitability was caused by a reduction of a regulatory influence during action potential firing, facilitating LAT neuronal activity. The effects of stress on excitability were occluded by agents that block calcium-activated potassium channels and reversed by pharmacological enhancement of calcium-activated potassium channels.
Conclusions: These data demonstrate a specific channelopathy that occurs in the amygdala after chronic stress. This enhanced excitability of amygdala neurons after chronic stress may explain the observed hyperresponsiveness of the amygdala in patients with posttraumatic stress disorder and may facilitate the emergence of depression or anxiety in other patients.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures

Comment in
-
Amygdala activity, fear, and anxiety: modulation by stress.Biol Psychiatry. 2010 Jun 15;67(12):1117-9. doi: 10.1016/j.biopsych.2010.04.027. Biol Psychiatry. 2010. PMID: 20525501 Free PMC article. No abstract available.
Similar articles
-
Increased Basolateral Amygdala Pyramidal Cell Excitability May Contribute to the Anxiogenic Phenotype Induced by Chronic Early-Life Stress.J Neurosci. 2015 Jul 1;35(26):9730-40. doi: 10.1523/JNEUROSCI.0384-15.2015. J Neurosci. 2015. PMID: 26134655 Free PMC article.
-
Pharmacological enhancement of calcium-activated potassium channel function reduces the effects of repeated stress on fear memory.Behav Brain Res. 2012 Jun 15;232(1):37-43. doi: 10.1016/j.bbr.2012.03.037. Epub 2012 Mar 31. Behav Brain Res. 2012. PMID: 22487247 Free PMC article.
-
Physiological role of calcium-activated potassium currents in the rat lateral amygdala.J Neurosci. 2002 Mar 1;22(5):1618-28. doi: 10.1523/JNEUROSCI.22-05-01618.2002. J Neurosci. 2002. PMID: 11880492 Free PMC article.
-
Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons.J Neurosci. 2008 Mar 19;28(12):3209-20. doi: 10.1523/JNEUROSCI.4310-07.2008. J Neurosci. 2008. PMID: 18354024 Free PMC article.
-
The Ca2+-activated K+ channel of intermediate conductance:a possible target for immune suppression.Expert Opin Ther Targets. 2002 Dec;6(6):623-36. doi: 10.1517/14728222.6.6.623. Expert Opin Ther Targets. 2002. PMID: 12472376 Review.
Cited by
-
Genomic glucocorticoid receptor effects guide acute stress-induced delayed anxiety and basolateral amygdala spine plasticity in rats.Neurobiol Stress. 2023 Nov 10;28:100587. doi: 10.1016/j.ynstr.2023.100587. eCollection 2024 Jan. Neurobiol Stress. 2023. PMID: 38075022 Free PMC article.
-
Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure.Mol Psychiatry. 2013 Oct;18(10):1125-35. doi: 10.1038/mp.2012.90. Epub 2012 Jul 10. Mol Psychiatry. 2013. PMID: 22776900 Free PMC article.
-
Central stress pathways in the development of cardiovascular disease.Clin Auton Res. 2024 Feb;34(1):99-116. doi: 10.1007/s10286-023-01008-x. Epub 2023 Dec 17. Clin Auton Res. 2024. PMID: 38104300 Review.
-
Amygdala connectivity related to subsequent stress responses during the COVID-19 outbreak.Front Psychiatry. 2023 Feb 23;14:999934. doi: 10.3389/fpsyt.2023.999934. eCollection 2023. Front Psychiatry. 2023. PMID: 36911118 Free PMC article.
-
Post-Traumatic Stress Disorder: The Relationship Between the Fear Response and Chronic Stress.Chronic Stress (Thousand Oaks). 2017 Jun 27;1:2470547017713297. doi: 10.1177/2470547017713297. eCollection 2017 Jan-Dec. Chronic Stress (Thousand Oaks). 2017. PMID: 32440579 Free PMC article. Review.
References
-
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol Bull. 2008;134:829–885. - PubMed
-
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats' anxiety- and depression-like behaviors in adulthood: The importance of stressor type and gender. Behav Neurosci. 2007;121:462–474. - PubMed
-
- Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci. 2008;122:282–292. - PubMed
-
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. - PubMed
-
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

