The integrin co-activator Kindlin-3 is expressed and functional in a non-hematopoietic cell, the endothelial cell
- PMID: 20378539
- PMCID: PMC2881789
- DOI: 10.1074/jbc.M109.085746
The integrin co-activator Kindlin-3 is expressed and functional in a non-hematopoietic cell, the endothelial cell
Abstract
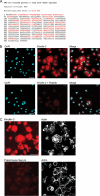
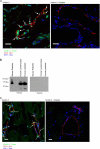
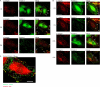
Integrin activation is crucial for numerous cellular responses, including cell adhesion, migration, and survival. Recent studies in mice have specifically emphasized the vital role of kindlin-3 in integrin activation. Kindlin-3 deficiency in humans also has now been documented and includes symptoms of bleeding, frequent infections, and osteopetrosis, which are consequences of an inability to activate beta1, beta2, and beta3 integrins. To date, kindlin-3 was thought to be restricted to hematopoietic cells. In this article, we demonstrate that kindlin-3 is present in human endothelial cells derived from various anatomical origins. The mRNA and protein for KINDLIN-3 was detected in endothelial cells by reverse transcription-PCR and Western blots. When subjected to sequencing by mass spectrometry, the protein was identified as authentic kindlin-3 and unequivocally distinguished from KINDLIN-1 and KINDLIN-2 or any other known protein. By quantitative real time PCR, the level of kindlin-3 in endothelial cells was 20-50% of that of kindlin-2. Using knockdown approaches, we show that kindlin-3 plays a role in integrin-mediated adhesion of endothelial cells. This function depends upon the integrin and substrate and is distinct from that of kindlin-2. Formation of tube-like structures in Matrigel also was impaired by kindlin-3 knockdown. Mechanistically, the distinct functions of the kindlins can be traced to differences in their subcellular localization in integrin-containing adhesion structures. Thus, the prevailing view that individual kindlins exert their functions in a cell type-specific manner must now be modified to consider distinct functions of the different family members within the same cell type.
Figures
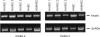



References
-
- Ma Y. Q., Qin J., Plow E. F. (2007) J. Thromb. Haemost. 5, 1345–1352 - PubMed
-
- Tu Y., Wu S., Shi X., Chen K., Wu C. (2003) Cell 113, 37–47 - PubMed
-
- Ussar S., Wang H. V., Linder S., Fässler R., Moser M. (2006) Exp. Cell Res. 312, 3142–3151 - PubMed
-
- Shi X., Ma Y. Q., Tu Y., Chen K., Wu S., Fukuda K., Qin J., Plow E. F., Wu C. (2007) J. Biol. Chem. 282, 20455–20466 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

