Gene therapy rescues cone function in congenital achromatopsia
- PMID: 20378608
- PMCID: PMC2883338
- DOI: 10.1093/hmg/ddq136
Gene therapy rescues cone function in congenital achromatopsia
Erratum in
- Hum Mol Genet. 2011 Dec 15;20(24):5024
Abstract
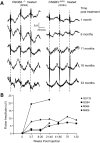
The successful restoration of visual function with recombinant adeno-associated virus (rAAV)-mediated gene replacement therapy in animals and humans with an inherited disease of the retinal pigment epithelium has ushered in a new era of retinal therapeutics. For many retinal disorders, however, targeting of therapeutic vectors to mutant rods and/or cones will be required. In this study, the primary cone photoreceptor disorder achromatopsia served as the ideal translational model to develop gene therapy directed to cone photoreceptors. We demonstrate that rAAV-mediated gene replacement therapy with different forms of the human red cone opsin promoter led to the restoration of cone function and day vision in two canine models of CNGB3 achromatopsia, a neuronal channelopathy that is the most common form of achromatopsia in man. The robustness and stability of the observed treatment effect was mutation independent, but promoter and age dependent. Subretinal administration of rAAV5-hCNGB3 with a long version of the red cone opsin promoter in younger animals led to a stable therapeutic effect for at least 33 months. Our results hold promise for future clinical trials of cone-directed gene therapy in achromatopsia and other cone-specific disorders.
Figures
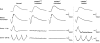


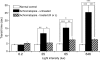

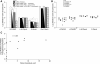
 .
.Similar articles
-
Safety and Efficacy of AAV5 Vectors Expressing Human or Canine CNGB3 in CNGB3-Mutant Dogs.Hum Gene Ther Clin Dev. 2017 Dec;28(4):197-207. doi: 10.1089/humc.2017.125. Epub 2017 Oct 11. Hum Gene Ther Clin Dev. 2017. PMID: 29020838 Free PMC article.
-
Transient photoreceptor deconstruction by CNTF enhances rAAV-mediated cone functional rescue in late stage CNGB3-achromatopsia.Mol Ther. 2013 Jun;21(6):1131-41. doi: 10.1038/mt.2013.50. Epub 2013 Apr 9. Mol Ther. 2013. PMID: 23568263 Free PMC article.
-
Cone-Specific Promoters for Gene Therapy of Achromatopsia and Other Retinal Diseases.Hum Gene Ther. 2016 Jan;27(1):72-82. doi: 10.1089/hum.2015.130. Hum Gene Ther. 2016. PMID: 26603570 Free PMC article.
-
Gene Therapy for Color Blindness.Yale J Biol Med. 2017 Dec 19;90(4):543-551. eCollection 2017 Dec. Yale J Biol Med. 2017. PMID: 29259520 Free PMC article. Review.
-
[Gene replacement therapy in achromatopsia type 2].Klin Monbl Augenheilkd. 2014 Mar;231(3):232-40. doi: 10.1055/s-0034-1368180. Epub 2014 Mar 21. Klin Monbl Augenheilkd. 2014. PMID: 24658860 Review. German.
Cited by
-
A comprehensive review of retinal gene therapy.Mol Ther. 2013 Mar;21(3):509-19. doi: 10.1038/mt.2012.280. Epub 2013 Jan 29. Mol Ther. 2013. PMID: 23358189 Free PMC article. Review.
-
Retinal morphology of patients with achromatopsia during early childhood: implications for gene therapy.JAMA Ophthalmol. 2014 Jul;132(7):823-31. doi: 10.1001/jamaophthalmol.2014.685. JAMA Ophthalmol. 2014. PMID: 24676353 Free PMC article.
-
A cyclic nucleotide-gated channel mutation associated with canine daylight blindness provides insight into a role for the S2 segment tri-Asp motif in channel biogenesis.PLoS One. 2014 Feb 21;9(2):e88768. doi: 10.1371/journal.pone.0088768. eCollection 2014. PLoS One. 2014. PMID: 24586388 Free PMC article.
-
Impact of gene therapy for canine monogenic diseases on the progress of preclinical studies.J Appl Genet. 2020 May;61(2):179-186. doi: 10.1007/s13353-020-00554-8. Epub 2020 Mar 18. J Appl Genet. 2020. PMID: 32189222 Free PMC article. Review.
-
Genetic and phenotypic variations of inherited retinal diseases in dogs: the power of within- and across-breed studies.Mamm Genome. 2012 Feb;23(1-2):40-61. doi: 10.1007/s00335-011-9361-3. Epub 2011 Nov 8. Mamm Genome. 2012. PMID: 22065099 Free PMC article. Review.
References
-
- Acland G.M., Aguirre G.D., Ray J., Zhang Q., Aleman T.S., Cideciyan A.V., Pearce-Kelling S.E., Anand V., Zeng Y., Maguire A.M., et al. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 2001;28:92–95. doi:10.1038/88327. - DOI - PubMed
-
- Acland G.M., Aguirre G.D., Bennett J., Aleman T.S., Cideciyan A.V., Bennicelli J., Dejneka N.S., Pearce-Kelling S.E., Maguire A.M., Palczewski K., et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol. Ther. 2005;12:1072–1082. doi:10.1016/j.ymthe.2005.08.008. - DOI - PMC - PubMed
-
- Pang J.J., Chang B., Kumar A., Nusinowitz S., Noorwez S.M., Li J., Rani A., Foster T.C., Chiodo V.A., Doyle T., et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol. Ther. 2006;13:565–572. doi:10.1016/j.ymthe.2005.09.001. - DOI - PubMed
-
- Hauswirth W.W., Aleman T.S., Kaushal S., Cideciyan A.V., Schwartz S.B., Wang L., Conlon T.J., Boye S.L., Flotte T.R., Byrne B.J., et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi:10.1089/hum.2008.107. - DOI - PMC - PubMed
-
- Cideciyan A.V., Aleman T.S., Boye S.L., Schwartz S.B., Kaushal S., Roman A.J., Pang J.J., Sumaroka A., Windsor E.A., Wilson J.M., et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl Acad. Sci. USA. 2008;105:15112–15117. doi:10.1073/pnas.0807027105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-EY013132/EY/NEI NIH HHS/United States
- R01-EY006855/EY/NEI NIH HHS/United States
- K12-EY015398/EY/NEI NIH HHS/United States
- P30-EY008571/EY/NEI NIH HHS/United States
- T32-EY007132/EY/NEI NIH HHS/United States
- R01 EY019304/EY/NEI NIH HHS/United States
- P30-EY001583/EY/NEI NIH HHS/United States
- R01-EY019304/EY/NEI NIH HHS/United States
- R01-EY011123/EY/NEI NIH HHS/United States
- R01-EY017549/EY/NEI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 EY006855/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

