An obligate role for membrane-associated neutral sphingomyelinase activity in orienting chemotactic migration of human neutrophils
- PMID: 20378749
- PMCID: PMC3049232
- DOI: 10.1165/rcmb.2010-0019OC
An obligate role for membrane-associated neutral sphingomyelinase activity in orienting chemotactic migration of human neutrophils
Abstract
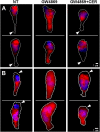
For polymorphonuclear neutrophils (PMNs) to orient migration to chemotactic gradients, weak external asymmetries must be amplified into larger internal signaling gradients. Lipid mediators, associated with the plasma membrane and within the cell, participate in generating these gradients. This study examined the role in PMN chemotaxis of neutral sphingomyelinase (N-SMase), a plasma membrane-associated enzyme that converts sphingomyelin to ceramide. A noncompetitive N-SMase inhibitor, GW4869 (5 mM, 5 minutes), did not inhibit PMN motility (as percentage of motile cells, or mean cell velocity), but it abrogated any orientation of movement toward the source of the chemotaxin, formylmethionylleucylphenylanaline (FMLP) (net displacement along the gradient axis in micrometers, or as percentage of total migration distance). This defect could be completely reversed by treatment with lignoceric ceramide (5 μg/ml, 15 minutes). Immunolocalization studies demonstrated that N-SMase (1) distributes preferentially toward the leading edge of some elongated cells, (2) is associated with the plasma membrane, (3) is more than 99.5% localized to the cytofacial aspect of the plasma membrane, (4) is excluded from pseudopodial extensions, and (5) increases rapidly in response to FMLP. Morphologically, the inhibition of N-SMase limited cellular spreading and the extension of sheet-like pseudopods. Elongated PMNs demonstrated a polarized distribution of GTPases, with Rac 1/2 accumulated at, and RhoA excluded from, the front of the cell. This polarity was negated by N-SMase inhibition and restored by lignoceric ceramide. We conclude that N-SMase at the cytofacial plasma membrane is an essential element for the proper orientation of PMNs in FMLP gradients, at least in part by polarizing the distribution of Rac 1/2 and RhoA GTPases.
Figures


Similar articles
-
Migrating human neutrophils exhibit dynamic spatiotemporal variation in membrane lipid organization.Am J Respir Cell Mol Biol. 2010 Oct;43(4):498-506. doi: 10.1165/rcmb.2009-0286OC. Epub 2009 Nov 20. Am J Respir Cell Mol Biol. 2010. PMID: 19933376 Free PMC article.
-
Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase.J Biol Chem. 2002 Oct 25;277(43):41128-39. doi: 10.1074/jbc.M206747200. Epub 2002 Aug 1. J Biol Chem. 2002. PMID: 12154098
-
Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis.Blood. 2006 Oct 15;108(8):2814-20. doi: 10.1182/blood-2006-01-010363. Epub 2006 Jun 29. Blood. 2006. PMID: 16809619 Free PMC article.
-
Ceramide and sphingomyelinases in the regulation of stress responses.Chem Phys Lipids. 1999 Nov;102(1-2):141-7. doi: 10.1016/s0009-3084(99)00082-1. Chem Phys Lipids. 1999. PMID: 11001568 Review.
-
The roles of neutral sphingomyelinases in neurological pathologies.Neurochem Res. 2012 Jun;37(6):1137-49. doi: 10.1007/s11064-011-0692-y. Epub 2012 Jan 12. Neurochem Res. 2012. PMID: 22237969 Review.
Cited by
-
The Regulation of Neutrophil Migration in Patients with Sepsis: The Complexity of the Molecular Mechanisms and Their Modulation in Sepsis and the Heterogeneity of Sepsis Patients.Cells. 2023 Mar 24;12(7):1003. doi: 10.3390/cells12071003. Cells. 2023. PMID: 37048076 Free PMC article. Review.
-
Differential Modulation of 25-hydroxycholecalciferol on Innate Immunity of Broiler Breeder Hens.Animals (Basel). 2021 Jun 10;11(6):1742. doi: 10.3390/ani11061742. Animals (Basel). 2021. PMID: 34200930 Free PMC article.
-
The Promotional Effect of GW4869 on C. albicans Invasion and Cellular Damage in a Murine Model of Oral Candidiasis.Pathogens. 2022 Dec 12;11(12):1522. doi: 10.3390/pathogens11121522. Pathogens. 2022. PMID: 36558856 Free PMC article.
-
Sphingomyelin Breakdown in T Cells: Role of Membrane Compartmentalization in T Cell Signaling and Interference by a Pathogen.Front Cell Dev Biol. 2019 Aug 13;7:152. doi: 10.3389/fcell.2019.00152. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31457008 Free PMC article. Review.
-
β-Glucan-stimulated neutrophil secretion of IL-1α is independent of GSDMD and mediated through extracellular vesicles.Cell Rep. 2021 May 18;35(7):109139. doi: 10.1016/j.celrep.2021.109139. Cell Rep. 2021. PMID: 34010648 Free PMC article.
References
-
- Affolter M, Weijer CJ. Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell 2005;9:19–34. - PubMed
-
- Kay RR, Langridge P, Traynor D, Hoeller O. Changing directions in the study of chemotaxis. Nat Rev Mol Cell Biol 2008;9:455–463. - PubMed
-
- Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res 2003;44:655–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

