Inhibition of histone deacetylase expands the renal progenitor cell population
- PMID: 20378823
- PMCID: PMC2865739
- DOI: 10.1681/ASN.2009080851
Inhibition of histone deacetylase expands the renal progenitor cell population
Abstract
One of the first hallmarks of kidney regeneration is the reactivation of genes normally required during organogenesis. Identification of chemicals with the potential to enhance this reactivation could therapeutically promote kidney regeneration. Here, we found that 4-(phenylthio)butanoic acid (PTBA) expanded the expression domains of molecular markers of kidney organogenesis in zebrafish. PTBA exhibits structural and functional similarity to the histone deacetylase (HDAC) inhibitors 4-phenylbutanoic acid and trichostatin A; treatment with these HDAC inhibitors also expanded the renal progenitor cell population. Analyses in vitro and in vivo confirmed that PTBA functions as an inhibitor of HDAC activity. Furthermore, PTBA-mediated renal progenitor cell expansion required retinoic acid signaling. In summary, these results support a mechanistic link among renal progenitor cells, HDAC, and the retinoid pathway. Whether PTBA holds promise as a therapeutic agent to promote renal regeneration requires further study.
Figures
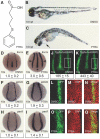
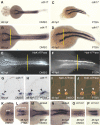


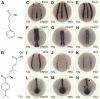


Comment in
-
From Proteus to Prometheus: learning from fish to modulate regeneration.J Am Soc Nephrol. 2010 May;21(5):726-8. doi: 10.1681/ASN.2010020228. Epub 2010 Apr 8. J Am Soc Nephrol. 2010. PMID: 20378822 No abstract available.
References
-
- Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC: High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol 1: 263–264, 2005. - PubMed
-
- Peterson RT, Shaw SY, Peterson TA, Milan DJ, Zhong TP, Schreiber SL, MacRae CA, Fishman MC: Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol 22: 595–599, 2004. - PubMed
-
- Stern HM, Murphey RD, Shepard JL, Amatruda JF, Straub CT, Pfaff KL, Weber G, Tallarico JA, King RW, Zon LI: Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat Chem Biol 1: 366–370, 2005. - PubMed
-
- Yeh JR, Munson KM, Chao YL, Peterson QP, Macrae CA, Peterson RT: AML1-ETO reprograms hematopoietic cell fate by downregulating scl expression. Development 135: 401–410, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

