Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction
- PMID: 20378859
- PMCID: PMC2885138
- DOI: 10.1161/CIRCRESAHA.109.212589
Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction
Abstract
Rationale: The success of cardiac stem cell therapies is limited by low cell retention, due at least in part to washout via coronary veins.
Objective: We sought to counter the efflux of transplanted cells by rendering them magnetically responsive and imposing an external magnetic field on the heart during and immediately after injection.
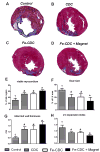
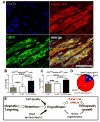
Methods and results: Cardiosphere-derived cells (CDCs) were labeled with superparamagnetic microspheres (SPMs). In vitro studies revealed that cell viability and function were minimally affected by SPM labeling. SPM-labeled rat CDCs were injected intramyocardially, with and without a superimposed magnet. With magnetic targeting, cells were visibly attracted toward the magnet and accumulated around the ischemic zone. In contrast, the majority of nontargeted cells washed out immediately after injection. Fluorescence imaging revealed more retention of transplanted cells in the heart, and less migration into other organs, in the magnetically targeted group. Quantitative PCR confirmed that magnetic targeting enhanced cell retention (at 24 hours) and engraftment (at 3 weeks) in the recipient hearts by approximately 3-fold compared to nontargeted cells. Morphometric analysis revealed maximal attenuation of left ventricular remodeling, and echocardiography showed the greatest functional improvement, in the magnetic targeting group. Histologically, more engrafted cells were evident with magnetic targeting, but there was no incremental inflammation.
Conclusions: Magnetic targeting enhances cell retention, engraftment and functional benefit. This novel method to improve cell therapy outcomes offers the potential for rapid translation into clinical applications.
Conflict of interest statement
Figures






References
-
- Wollert KC, Drexler H. Clinical Applications of Stem Cells for the Heart. Circ Res. 2005;96:151–163. - PubMed
-
- Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J, Barton PJR, Terracciano CMN, Yacoub MH, Suzuki K. Direct Intramyocardial But Not Intracoronary Injection of Bone Marrow Cells Induces Ventricular Arrhythmias in a Rat Chronic Ischemic Heart Failure Model. Circulation. 2007;115:2254–2261. - PubMed
-
- Ye L, Haider HK, Guo C, Sim EKW. Cell-Based VEGF Delivery Prevents Donor Cell Apoptosis After Transplantation. The Annals of Thoracic Surgery. 2007;83:1233–1234. - PubMed
-
- Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte Grafting for Cardiac Repair: Graft Cell Death and Anti-Death Strategies. Journal of Molecular and Cellular Cardiology. 2001;33:907–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

