Recovery from a DNA-damage-induced G2 arrest requires Cdk-dependent activation of FoxM1
- PMID: 20379221
- PMCID: PMC2892325
- DOI: 10.1038/embor.2010.46
Recovery from a DNA-damage-induced G2 arrest requires Cdk-dependent activation of FoxM1
Abstract
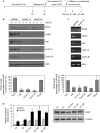
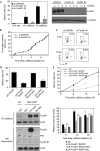
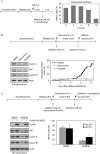
Activation of the DNA-damage checkpoint culminates in the inhibition of cyclin-dependent kinase (Cdk) complexes to prevent cell-cycle progression. We have shown recently that Cdk activity is required for activation of the Forkhead transcription factor FoxM1, an important regulator of gene expression in the G2 phase of the cell cycle. Here, we show that FoxM1 is transcriptionally active during a DNA-damage-induced G2 arrest and is essential for checkpoint recovery. Paradoxically, Cdk activity, although reduced after checkpoint activation, is required to maintain FoxM1-dependent transcription during the arrest and for expression of pro-mitotic targets such as cyclin A, cyclin B and Plk1. Indeed, we find that cells need to retain sufficient levels of Cdk activity during the DNA-damage response to maintain cellular competence to recover from a DNA-damaging insult.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Comment in
-
Checkpoint recovery after DNA damage: a rolling stop for CDKs.EMBO Rep. 2010 Jun;11(6):411-2. doi: 10.1038/embor.2010.76. Epub 2010 May 14. EMBO Rep. 2010. PMID: 20467435 Free PMC article.
References
-
- Bartek J, Lukas J (2007) DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol 19: 238–245 - PubMed
-
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 - PubMed
-
- Chae HD, Yun J, Bang YJ, Shin DY (2004) Cdk2-dependent phosphorylation of the NF-Y transcription factor is essential for the expression of the cell cycle-regulatory genes and cell cycle G1/S and G2/M transitions. Oncogene 23: 4084–4088 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

