Low-power ultrasounds as a tool to culture human osteoblasts inside cancellous hydroxyapatite
- PMID: 20379359
- PMCID: PMC2850136
- DOI: 10.1155/2010/456240
Low-power ultrasounds as a tool to culture human osteoblasts inside cancellous hydroxyapatite
Abstract
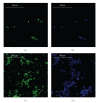
Bone graft substitutes and cancellous biomaterials have been widely used to heal critical-size long bone defects due to trauma, tumor resection, and tissue degeneration. In particular, porous hydroxyapatite is widely used in reconstructive bone surgery owing to its biocompatibility. In addition, the in vitro modification of cancellous hydroxyapatite with osteogenic signals enhances the tissue regeneration in vivo, suggesting that the biomaterial modification could play an important role in tissue engineering. In this study, we have followed a tissue-engineering strategy where ultrasonically stimulated SAOS-2 human osteoblasts proliferated and built their extracellular matrix inside a porous hydroxyapatite scaffold. The ultrasonic stimulus had the following parameters: average power equal to 149 mW and frequency of 1.5 MHz. In comparison with control conditions, the ultrasonic stimulus increased the cell proliferation and the surface coating with bone proteins (decorin, osteocalcin, osteopontin, type-I collagen, and type-III collagen). The mechanical stimulus aimed at obtaining a better modification of the biomaterial internal surface in terms of cell colonization and coating with bone matrix. The modified biomaterial could be used, in clinical applications, as an implant for bone repair.
Figures



Similar articles
-
In vitro electromagnetically stimulated SAOS-2 osteoblasts inside porous hydroxyapatite.J Biomed Mater Res A. 2010 Jun 15;93(4):1272-9. doi: 10.1002/jbm.a.32620. J Biomed Mater Res A. 2010. PMID: 19827111 Free PMC article.
-
Use of a gelatin cryogel as biomaterial scaffold in the differentiation process of human bone marrow stromal cells.Annu Int Conf IEEE Eng Med Biol Soc. 2010;2010:247-50. doi: 10.1109/IEMBS.2010.5627475. Annu Int Conf IEEE Eng Med Biol Soc. 2010. PMID: 21096747
-
Physically enhanced coating of a titanium plasma-spray surface with human SAOS-2 osteoblasts and extracellular matrix.Annu Int Conf IEEE Eng Med Biol Soc. 2007;2007:6415-8. doi: 10.1109/IEMBS.2007.4353824. Annu Int Conf IEEE Eng Med Biol Soc. 2007. PMID: 18003490
-
Applications of X-ray computed tomography for the evaluation of biomaterial-mediated bone regeneration in critical-sized defects.J Microsc. 2020 Mar;277(3):179-196. doi: 10.1111/jmi.12844. Epub 2019 Nov 20. J Microsc. 2020. PMID: 31701530 Review.
-
Biologically Inspired Collagen/Apatite Composite Biomaterials for Potential Use in Bone Tissue Regeneration-A Review.Materials (Basel). 2020 Apr 9;13(7):1748. doi: 10.3390/ma13071748. Materials (Basel). 2020. PMID: 32283608 Free PMC article. Review.
Cited by
-
A comparison of epithelial cells, fibroblasts, and osteoblasts in dental implant titanium topographies.Bioinorg Chem Appl. 2012;2012:687291. doi: 10.1155/2012/687291. Epub 2012 Jan 12. Bioinorg Chem Appl. 2012. PMID: 22287942 Free PMC article.
-
In Vitro Production of Calcified Bone Matrix onto Wool Keratin Scaffolds via Osteogenic Factors and Electromagnetic Stimulus.Materials (Basel). 2020 Jul 8;13(14):3052. doi: 10.3390/ma13143052. Materials (Basel). 2020. PMID: 32650489 Free PMC article.
-
Model of Murine Ventricular Cardiac Tissue for In Vitro Kinematic-Dynamic Studies of Electromagnetic and β-Adrenergic Stimulation.J Healthc Eng. 2017;2017:4204085. doi: 10.1155/2017/4204085. Epub 2017 Aug 8. J Healthc Eng. 2017. PMID: 29065600 Free PMC article.
-
Preparation and characterization of an advanced medical device for bone regeneration.AAPS PharmSciTech. 2014 Feb;15(1):75-82. doi: 10.1208/s12249-013-0033-3. Epub 2013 Oct 22. AAPS PharmSciTech. 2014. PMID: 24146118 Free PMC article.
-
High-Frequency Vibration Treatment of Human Bone Marrow Stromal Cells Increases Differentiation toward Bone Tissue.Bone Marrow Res. 2013;2013:803450. doi: 10.1155/2013/803450. Epub 2013 Mar 25. Bone Marrow Res. 2013. PMID: 23585968 Free PMC article.
References
-
- Nishikawa M, Myoui A, Ohgushi H, Ikeuchi M, Tamai N, Yoshikawa H. Bone tissue engineering using novel interconnected porous hydroxyapatite ceramics combined with marrow mesenchymal cells: quantitative and three-dimensional image analysis. Cell Transplantation. 2004;13(4):367–376. - PubMed
-
- Mauney JR, Blumberg J, Pirun M, Volloch V, Vunjak-Novakovic G, Kaplan DL. Osteogenic differentiation of human bone marrow stromal cells on partially demineralized bone scaffolds in vitro. Tissue Engineering. 2004;10(1-2):81–92. - PubMed
-
- Devin JE, Attawia MA, Laurencin CT, et al. Three-dimensional degradable porous polymer-ceramic matrices for use in bone repair. Journal of Biomaterials Science, Polymer Edition. 1996;7(8):661–669. - PubMed
-
- Deb S, Mandegaran R, Di Silvio L. A porous scaffold for bone tissue engineering/45S5 Bioglass® derived porous scaffolds for co-culturing osteoblasts and endothelial cells. Journal of Materials Science: Materials in Medicine. 2010;21(3):893–905. - PubMed
-
- Francis L, Meng D, Knowles JC, Roy I, Boccaccini AR. Multi-functional P(3HB) microsphere/45S5 Bioglass®-based composite scaffolds for bone tissue engineering. Acta Biomaterialia. In press. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

