The role of the lactadherin in promoting intestinal DCs development in vivo and vitro
- PMID: 20379374
- PMCID: PMC2850507
- DOI: 10.1155/2010/357541
The role of the lactadherin in promoting intestinal DCs development in vivo and vitro
Abstract
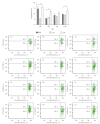
Lactadherin, as one of the immune components in the breast milk, might play a role in the intestinal immune system of newborn. Therefore, we investigated the effect of lactadherin-feeding in early time on the development of intestinal immune system compared with naturally rearing and artificially rearing (non-lactadherin). In the present study, we observed that the Peyer's Patches (PP) from the pups of artificially reared group with lactadherin added were characterized by an excess of OX62(+)CD4(+)SIRP(+) DC cells and a higher expression of CD3(+)CD4(+)CD25(+)T cells. Additionally, this study also demonstrated that IL-10 production was dramatically increased when lactadherin was present in culture medium compared with lactadherin-absent culture. These results suggested that lactadherin could adjust intestinal DCs activity, induce CD3(+)CD4(+)CD25(+)T cell differentiation, and enhance IL-10 production.
Figures

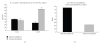
References
-
- Newburg DS. Bioactive components of human milk: evolution, efficiency, and protection. Advances in Experimental Medicine and Biology. 2001;501:3–10. - PubMed
-
- Grulee CG, Sanford HN, Herron PH. Breast and artificial feeding: influence on morbidity and mortality of twenty thousand infants. Journal of the American Medical Association. 1934;103:735–738.
-
- Hanson LÅ, Ceafalau L, Mattsby-Baltzer I, et al. The mammary gland—infant intestine immunologic dyad. Advances in Experimental Medicine and Biology. 2000;478:65–76. - PubMed
-
- Boudry G, Péron V, Le Huërou-Luron I, Lallès JP, Sève B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. Journal of Nutrition. 2004;134(9):2256–2262. - PubMed
-
- Teichberg S, Isolauri E, Wapnir RA, Roberts B, Lifshitz F. Development of the neonatal rat small intestinal barrier to nonspecific macromolecular absorption: effect of early weaning to artificial diets. Pediatric Research. 1990;28(1):31–37. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

