Biograft-HT as a bone graft material in the treatment of periodontal vertical defects and its clinical and radiological evaluation: Clinical study
- PMID: 20379411
- PMCID: PMC2848784
- DOI: 10.4103/0972-124X.60226
Biograft-HT as a bone graft material in the treatment of periodontal vertical defects and its clinical and radiological evaluation: Clinical study
Abstract
Aim: To determine the efficacy of Biograft-HT((R)) as a bone graft material in the treatment of vertical defects in generalized chronic periodontitis patients and their clinical and radiological evaluation.
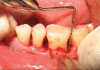
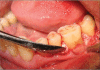
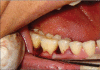
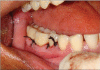
Patients and methods: Twenty patients diagnosed with generalized chronic periodontitis having two or more vertical defects were selected for this study. Clinical parameters like plaque index, gingival index, probing pocket depth and clinical attachment levels were recorded at different points of time over six months. Radiographic evaluation included the depth of the bone defect and the percentage of bone defect fill and was carried out for both the groups at baseline, three months and six months. After recording clinical parameters and administering phase-1 therapy, the sites were randomly treated either with Biograft- HT((R)) or open flap debridement only.
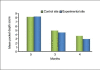
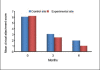
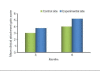
Results: At the end of six months there was a significant reduction in the plaque and gingival scores in both test and control groups. There was 64% decrease in probing pocket depth for the test site as compared to 54.52% decrease seen for the control group. Similarly there was an 84.82% gain in clinical attachment level from the baseline to six months post operatively for the experimental group in comparison to 68.83% gain for the control group. Furthermore, 43.57% bone fill was observed for the experimental site whereas only 17.98% of bone fill was evident in the control site.
Conclusion: Biograft -HT improves healing outcomes, leads to a reduction of probing depth, a resolution of osseous defects and a gain in clinical attachment, compared with open flap debridement by itself.
Keywords: Beta-triclacium phosphate; hydroxyapatite; vertical defects.
Conflict of interest statement
Figures







Similar articles
-
A Clinical and Radiographic Evaluation of Periodontal Regenerative Potential of PerioGlas®: A Synthetic, Resorbable Material in Treating Periodontal Infrabony Defects.J Int Oral Health. 2014 Jun;6(3):20-6. Epub 2014 Jun 26. J Int Oral Health. 2014. PMID: 25083028 Free PMC article.
-
Evaluation of biphasic hydroxapatite and β-tricalcium phosphate as a bone graft material in the treatment of periodontal vertical bony defects - A clinical and digital radiological measurement study.Indian J Dent Res. 2022 Apr-Jun;33(2):152-157. doi: 10.4103/ijdr.IJDR_234_19. Indian J Dent Res. 2022. PMID: 36254951
-
A novel marginal periosteal pedicle graft as an autogenous guided tissue membrane for the treatment of intrabony periodontal defects.J Int Acad Periodontol. 2008 Oct;10(4):106-17. J Int Acad Periodontol. 2008. PMID: 19055224 Clinical Trial.
-
Evaluation of HTR polymer (Bioplant HTR) as a bone graft material in the treatment of interproximal vertical bony defects: a clinical and radiological study.Indian J Dent Res. 2010 Apr-Jun;21(2):179-84. doi: 10.4103/0970-9290.66630. Indian J Dent Res. 2010. PMID: 20657084 Clinical Trial.
-
Clinical and radiographic evaluation of regenerative potential of GTR membrane (Biomesh®) along with alloplastic bone graft (Biograft®) in the treatment of periodontal intrabony defects.J Contemp Dent Pract. 2013 May 1;14(3):434-9. doi: 10.5005/jp-journals-10024-1340. J Contemp Dent Pract. 2013. PMID: 24171985 Clinical Trial.
Cited by
-
Role of platelet-rich plasma in combination with alloplastic bone substitute in regeneration of osseous defects.J Oral Biol Craniofac Res. 2011 Oct-Dec;1(1):17-23. doi: 10.1016/S2212-4268(11)60006-7. J Oral Biol Craniofac Res. 2011. PMID: 25756013 Free PMC article.
-
Assessment of Biograft-HT with I-PRF Graft in Immediate Dental Implant Placement.J Pharm Bioallied Sci. 2023 Jul;15(Suppl 2):S1168-S1170. doi: 10.4103/jpbs.jpbs_178_23. Epub 2023 Apr 28. J Pharm Bioallied Sci. 2023. PMID: 37693995 Free PMC article.
-
Systematic Review Regarding the Clinical Implications of Allograft and Alloplastic Bone Substituents Used for Periodontal Regenerative Therapy.J Clin Med. 2025 Jan 29;14(3):894. doi: 10.3390/jcm14030894. J Clin Med. 2025. PMID: 39941565 Free PMC article. Review.
-
The use of hydroxyapatite bone substitute grafting for alveolar ridge preservation, sinus augmentation, and periodontal bone defect: A systematic review.Heliyon. 2018 Nov 2;4(10):e00884. doi: 10.1016/j.heliyon.2018.e00884. eCollection 2018 Oct. Heliyon. 2018. PMID: 30417149 Free PMC article.
References
-
- Carranaza FA, Newman MG. Reconstructive osseous surgery. 8th ed. Philadelphia, USA: WB Saunders Company; 1999. Clinical Periodontology; pp. 622–39.
-
- Reynolds MA, Aichelmann-Reidy ME, Branch Mays GI, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. Ann Periodontol. 2003;8:227–65. - PubMed
-
- Nasr HF, Aichelmann Reidy, Yukna RA. Bone and bone substitutes. Periodontology 2000. 1999;19:74–86. - PubMed
-
- Stahl SS, Froum S. Human intrabony lesion responses to debridement, porous hydroxyapatite implants and teflon barrier membranes. J Clin Periodontol. 1991;18:605–10. - PubMed
-
- Kohad RM, Shetty S, Yeltiwar RK, Vaidya SN. A new synthetic hydroxyapatite – the right answer to bone regeneration. J Ind Soc Periodontol. 2001;4:6–11.
LinkOut - more resources
Full Text Sources
Other Literature Sources

