Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage
- PMID: 20380694
- PMCID: PMC2873506
- DOI: 10.1186/1742-4690-7-32
Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage
Abstract
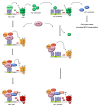
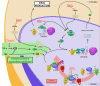
The introduction of the highly active antiretroviral therapy (HAART) has greatly improved survival. However, these treatments fail to definitively cure the patients and unveil the presence of quiescent HIV-1 reservoirs like cells from monocyte-macrophage lineage. A purge, or at least a significant reduction of these long lived HIV-1 reservoirs will be needed to raise the hope of the viral eradication. This review focuses on the molecular mechanisms responsible for viral persistence in cells of the monocyte-macrophage lineage. Controversy on latency and/or cryptic chronic replication will be specifically evoked. In addition, since HIV-1 infected monocyte-macrophage cells appear to be more resistant to apoptosis, this obstacle to the viral eradication will be discussed. Understanding the intimate mechanisms of HIV-1 persistence is a prerequisite to devise new and original therapies aiming to achieve viral eradication.
Figures

References
-
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. - DOI - PubMed
-
- Di Mascio M, Dornadula G, Zhang H, Sullivan J, Xu Y, Kulkosky J, Pomerantz RJ, Perelson AS. In a subset of subjects on highly active antiretroviral therapy, human immunodeficiency virus type 1 RNA in plasma decays from 50 to <5 copies per milliliter, with a half-life of 6 months. J Virol. 2003;77:2271–2275. doi: 10.1128/JVI.77.3.2271-2275.2003. - DOI - PMC - PubMed
-
- Dornadula G, Zhang H, VanUitert B, Stern J, Livornese L Jr, Ingerman MJ, Witek J, Kedanis RJ, Natkin J, DeSimone J, Pomerantz RJ. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. Jama. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

