Spinal microglial motility is independent of neuronal activity and plasticity in adult mice
- PMID: 20380706
- PMCID: PMC2857828
- DOI: 10.1186/1744-8069-6-19
Spinal microglial motility is independent of neuronal activity and plasticity in adult mice
Abstract
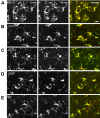
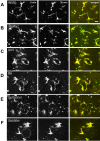
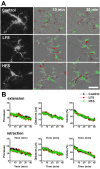
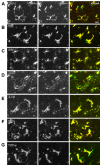
Microglia are the resident macrophages in the central nervous system. In the spinal cord dorsal horn, microglia stay in resting condition during physiological sensory processing, and are activated under pathological conditions such as peripheral nerve injury. In cases such as this, the nearby resting microglia increase their motility and accumulate at the site of injury. However, direct evidence to support that nerve activity can enhance the motility of microglia has not yet to be reported. In this study we investigated whether the activation of spinal microglia under in vivo nerve injury may be mimicked by neuronal activity in the spinal cord slice preparation. We found that local application of spinal excitatory neurotransmitters, such as glutamate and substance P did not cause any change in the motility of microglial cells in the spinal cord dorsal horn. The motility of microglial cells is unlikely modulated by other transmitters, neuromodulators and chemokines, because similar applications such as GABA, serotonin, noradrenaline, carbachol, fractalkine or interleukin did not produce any obvious effect. Furthermore, low or high frequency stimulation of spinal dorsal root fibers at noxious intensities failed to cause any enhanced extension or retraction of the microglia processes. By contrast, focal application of ATP triggered rapid and robust activation of microglial cells in the spinal dorsal horn. Our results provide the first evidence that the activation of microglia in the spinal cord after nerve injury is unlikely due solely to neuronal activity, non-neuronal factors are likely responsible for the activation of nerve injury-related microglial cells in the spinal dorsal horn.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

