Epstein-Barr virus BRLF1 inhibits transcription of IRF3 and IRF7 and suppresses induction of interferon-beta
- PMID: 20381110
- PMCID: PMC2871977
- DOI: 10.1016/j.virol.2010.03.014
Epstein-Barr virus BRLF1 inhibits transcription of IRF3 and IRF7 and suppresses induction of interferon-beta
Abstract
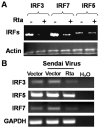
Activation of interferon regulatory factors (IRFs) 3 and 7 is essential for the induction of Type I interferons (IFN) and innate antiviral responses, and herpesviruses have evolved mechanisms to evade such responses. We previously reported that Epstein-Barr virus BZLF1, an immediate-early (IE) protein, inhibits the function of IRF7, but the role of BRLF1, the other IE transactivator, in IRF regulation has not been examined. We now show that BRLF1 expression decreased induction of IFN-beta, and reduced expression of IRF3 and IRF7; effects were dependent on N- and C-terminal regions of BRLF1 and its nuclear localization signal. Endogenous IRF3 and IRF7 RNA and protein levels were also decreased during cytolytic EBV infection. Finally, production of IFN-beta was decreased during lytic EBV infection and was associated with increased susceptibility to superinfection with Sendai virus. These data suggest a new role for BRLF1 with the ability to evade host innate immune responses.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Abele R, Tampe R. The ABCs of immunology: structure and function of TAP, the transporter associated with antigen processing. Physiology (Bethesda) 2004;19:216–24. - PubMed
-
- Adamson AL, Darr D, Holley-Guthrie E, Johnson RA, Mauser A, Swenson J, Kenney S. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J Virol. 2000;74(3):1224–33. - PMC - PubMed
-
- Barnes B, Lubyova B, Pitha PM. On the role of IRF in host defense. J Interferon Cytokine Res. 2002;22(1):59–71. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

