Interleukin-15 expression is increased in human eosinophilic esophagitis and mediates pathogenesis in mice
- PMID: 20381491
- PMCID: PMC4116278
- DOI: 10.1053/j.gastro.2010.03.057
Interleukin-15 expression is increased in human eosinophilic esophagitis and mediates pathogenesis in mice
Abstract
Background & aims: Quantitative microarray analyses have shown increased expression of interleukin-15 (IL-15) messenger RNA in the esophagus of patients with eosinophilic esophagitis (EoE), a recently recognized allergic disorder with poorly understood pathogenesis.
Methods: Quantitative polymerase chain reaction and enzyme-linked immunosorbent assay analyses were performed to examine protein and transcript levels in tissue samples from patients with EoE. Tissues from IL-15Ra-deficient and wild-type (control) mice were also examined. Tissue eosinophilia was determined by immunostaining for major basic protein and flow cytometry for cell-surface receptors.
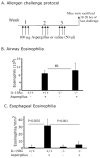
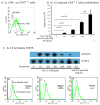
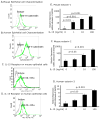
Results: Quantitative polymerase chain reaction analyses showed that levels of IL-15 and its receptor IL-15Ra were increased approximately 6- and approximately 10-fold, respectively, in tissues from patients with EoE and approximately 3- and approximately 4-fold, respectively, in mice with allergen-induced EoE. A >2-fold increase in serum IL-15 protein levels was also detected in human EoE samples compared with those from healthy individuals. Human IL-15 messenger RNA levels correlated with esophageal eosinophilia (P < .001). IL-15Ra-deficient mice were protected from allergen-induced esophageal eosinophilia compared with controls (P < .001), even though similar levels of airway eosinophilia were observed in all mice. IL-15 activated STAT5 and CD4(+) T cells to produce cytokines that act on eosinophils. Incubation of primary esophageal epithelial cells from mice and humans with IL-15 caused a dose-dependent increase in the mRNA expression and protein levels of eotaxin-1, -2, and -3.
Conclusions: IL-15 mediates in the pathogenesis of EoE. IL-15 activates CD4(+) T cells to produce cytokines that act on eosinophils.
Copyright 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
There are no conflicts of interest to disclose for all authors except Dr. Rothenberg who discloses consultancy relationships with Merck, Ception Therapueutics, Nycomed, Biocryst Pharmaceuticals, and Centocor.
Figures





Similar articles
-
Pathogenesis of allergen-induced eosinophilic esophagitis is independent of interleukin (IL)-13.Immunol Cell Biol. 2013 Jul;91(6):408-15. doi: 10.1038/icb.2013.21. Epub 2013 May 21. Immunol Cell Biol. 2013. PMID: 23689305 Free PMC article.
-
Elevated IL-33 expression is associated with pediatric eosinophilic esophagitis, and exogenous IL-33 promotes eosinophilic esophagitis development in mice.Am J Physiol Gastrointest Liver Physiol. 2016 Jan 1;310(1):G13-25. doi: 10.1152/ajpgi.00290.2015. Epub 2015 Oct 29. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26514775
-
Allergic skin sensitization promotes eosinophilic esophagitis through the IL-33-basophil axis in mice.J Allergy Clin Immunol. 2016 Nov;138(5):1367-1380.e5. doi: 10.1016/j.jaci.2016.02.034. Epub 2016 Apr 19. J Allergy Clin Immunol. 2016. PMID: 27233150
-
Biological Therapies for Eosinophilic Esophagitis: Where Do We Stand?Clin Rev Allergy Immunol. 2018 Oct;55(2):205-216. doi: 10.1007/s12016-018-8674-3. Clin Rev Allergy Immunol. 2018. PMID: 29372536 Review.
-
Eosinophilic esophagitis.Allergy Asthma Proc. 2019 Nov 1;40(6):462-464. doi: 10.2500/aap.2019.40.4272. Allergy Asthma Proc. 2019. PMID: 31690395 Review.
Cited by
-
T cell co-stimulatory molecules: a co-conspirator in the pathogenesis of eosinophilic esophagitis?Dig Dis Sci. 2013 Jun;58(6):1497-506. doi: 10.1007/s10620-013-2599-8. Epub 2013 Mar 2. Dig Dis Sci. 2013. PMID: 23456499 Review.
-
Type 2 Inflammation in Eosinophilic Esophagitis: From Pathophysiology to Therapeutic Targets.Front Physiol. 2022 Jan 12;12:815842. doi: 10.3389/fphys.2021.815842. eCollection 2021. Front Physiol. 2022. PMID: 35095572 Free PMC article. Review.
-
Allergic mechanisms in eosinophilic esophagitis.Gastroenterol Clin North Am. 2014 Jun;43(2):281-96. doi: 10.1016/j.gtc.2014.02.006. Epub 2014 Mar 22. Gastroenterol Clin North Am. 2014. PMID: 24813516 Free PMC article. Review.
-
Allergic mechanisms of Eosinophilic oesophagitis.Best Pract Res Clin Gastroenterol. 2015 Oct;29(5):709-720. doi: 10.1016/j.bpg.2015.09.012. Epub 2015 Sep 11. Best Pract Res Clin Gastroenterol. 2015. PMID: 26552770 Free PMC article. Review.
-
Discovery and characterization of a novel humanized anti-IL-15 antibody and its relevance for the treatment of refractory celiac disease and eosinophilic esophagitis.MAbs. 2017 Aug/Sep;9(6):927-944. doi: 10.1080/19420862.2017.1332553. Epub 2017 Jun 5. MAbs. 2017. PMID: 28581883 Free PMC article.
References
-
- Dahms BB. Reflux esophagitis: sequelae and differential diagnosis in infants and children including eosinophilic esophagitis. Pediatr Dev Pathol. 2004;7:5–16. - PubMed
-
- Kirsch R, Bokhary R, Marcon MA, Cutz E. Activated mucosal mast cells differentiate eosinophilic (allergic) esophagitis from gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2007;44:20–6. - PubMed
-
- Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc. 2005;61:795–801. - PubMed
-
- Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol. 2001;108:891–894. - PubMed
-
- Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

