Gene-chemical interactions in the developing mammalian nervous system: Effects on proliferation, neurogenesis and differentiation
- PMID: 20381523
- PMCID: PMC2934892
- DOI: 10.1016/j.neuro.2010.03.007
Gene-chemical interactions in the developing mammalian nervous system: Effects on proliferation, neurogenesis and differentiation
Abstract
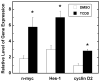
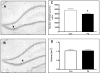
The orderly formation of the nervous system requires a multitude of complex, integrated and simultaneously occurring processes. Neural progenitor cells expand through proliferation, commit to different cell fates, exit the cell cycle, generate different neuronal and glial cell types, and new neurons migrate to specified areas and establish synaptic connections. Gestational and perinatal exposure to environmental toxicants, pharmacological agents and drugs of abuse produce immediate, persistent or late-onset alterations in behavioral, cognitive, sensory and/or motor functions. These alterations reflect the disruption of the underlying processes of CNS formation and development. To determine the neurotoxic mechanisms that underlie these deficits it is necessary to analyze and dissect the complex molecular processes that occur during the proliferation, neurogenesis and differentiation of cells. This symposium will provide a framework for understanding the orchestrated events of neurogenesis, the coordination of proliferation and cell fate specification by selected genes, and the effects of well-known neurotoxicants on neurogenesis in the retina, hippocampus and cerebellum. These three tissues share common developmental profiles, mediate diverse neuronal activities and function, and thus provide important substrates for analysis. This paper summarizes four invited talks that were presented at the 12th International Neurotoxicology Association meeting held in Jerusalem, Israel during the summer of 2009. Donald A. Fox described the structural and functional alterations following low-level gestational lead exposure in children and rodents that produced a supernormal electroretinogram and selective increases in neurogenesis and cell proliferation of late-born retinal neurons (rod photoreceptors and bipolar cells), but not Müller glia cells, in mice. Lisa Opanashuk discussed how dioxin [TCDD] binding to the arylhydrocarbon receptor [AhR], a transcription factor that regulates xenobiotic metabolizing enzymes and growth factors, increased granule cell formation and apoptosis in the developing mouse cerebellum. Alex Zharkovsky described how postnatal early postnatal lead exposure decreased cell proliferation, neurogenesis and gene expression in the dentate gyrus of the adult hippocampus and its resultant behavioral effects. Bernard Weiss illustrated how environmental endocrine disruptors produced age- and sex-dependent alterations in synaptogenesis and cognitive behavior.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICTS OF INTEREST
None of the authors has competing financial interests.
Figures

Similar articles
-
Increased proliferation of late-born retinal progenitor cells by gestational lead exposure delays rod and bipolar cell differentiation.Mol Vis. 2016 Dec 24;22:1468-1489. eCollection 2016. Mol Vis. 2016. PMID: 28050121 Free PMC article.
-
Low-level gestational lead exposure increases retinal progenitor cell proliferation and rod photoreceptor and bipolar cell neurogenesis in mice.Environ Health Perspect. 2011 Jan;119(1):71-7. doi: 10.1289/ehp.1002524. Epub 2010 Sep 14. Environ Health Perspect. 2011. PMID: 20840909 Free PMC article.
-
Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory.J Neurochem. 2013 May;125(3):430-45. doi: 10.1111/jnc.12130. Epub 2013 Jan 7. J Neurochem. 2013. PMID: 23240617 Free PMC article.
-
The Aryl Hydrocarbon Receptor and the Nervous System.Int J Mol Sci. 2018 Aug 24;19(9):2504. doi: 10.3390/ijms19092504. Int J Mol Sci. 2018. PMID: 30149528 Free PMC article. Review.
-
Social Cues, Adult Neurogenesis, and Reproductive Behavior.In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 13. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 13. PMID: 24830028 Free Books & Documents. Review.
Cited by
-
3-methylcholanthrene induces neurotoxicity in developing neurons derived from human CD34+Thy1+ stem cells by activation of aryl hydrocarbon receptor.Neuromolecular Med. 2013 Sep;15(3):570-92. doi: 10.1007/s12017-013-8243-0. Epub 2013 Jul 12. Neuromolecular Med. 2013. PMID: 23846855
-
Methylmercury: a potential environmental risk factor contributing to epileptogenesis.Neurotoxicology. 2012 Jan;33(1):119-26. doi: 10.1016/j.neuro.2011.12.014. Epub 2011 Dec 22. Neurotoxicology. 2012. PMID: 22206970 Free PMC article. Review.
-
Heated indoor swimming pools, infants, and the pathogenesis of adolescent idiopathic scoliosis: a neurogenic hypothesis.Environ Health. 2011 Oct 5;10:86. doi: 10.1186/1476-069X-10-86. Environ Health. 2011. PMID: 21975145 Free PMC article.
References
-
- Osman K, Pawlas K, Schutz A, Gazdik M, Sokal JA, Vahter M. Lead exposure and hearing effects in children in Katowice, Poland. Environ Res. 1999;80:1–8. - PubMed
-
- Rothenberg SJ, Poblano A, Schnaas L. Brainstem auditory evoked response at five years and prenatal and postnatal blood lead. Neurotoxicol Teratol. 2000;22:503–510. - PubMed
-
- Wasserman GA, Liu X, Popovac D, Factor-Litvak P, Kline J, Waternaux C, et al. The Yugoslavia Prospective Lead Study: contributions of prenatal and postnatal lead exposure to early intelligence. Neurotoxicol Teratol. 2000;22:811–818. - PubMed
-
- Altmann L, Sveinsson K, Kramer U, Weishoff-Houben M, Turfeld M, Winneke G, et al. Visual function in 6-year old children living in relation to lead and mercury levels. Neurotoxicol Teratol. 1998;20:9–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 EY007551/EY/NEI NIH HHS/United States
- EY07551/EY/NEI NIH HHS/United States
- R01ES016357/ES/NIEHS NIH HHS/United States
- R01ES012482/ES/NIEHS NIH HHS/United States
- ES07026/ES/NIEHS NIH HHS/United States
- R01 ES016357/ES/NIEHS NIH HHS/United States
- T32 ES007026/ES/NIEHS NIH HHS/United States
- R01 ES012482/ES/NIEHS NIH HHS/United States
- R01ES015509/ES/NIEHS NIH HHS/United States
- ES01247/ES/NIEHS NIH HHS/United States
- T32 EY007024/EY/NEI NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
- R01 ES007026/ES/NIEHS NIH HHS/United States
- R21 ES015509/ES/NIEHS NIH HHS/United States
- R21ES013512/ES/NIEHS NIH HHS/United States
- R21 ES013512/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources

