Signaling pathways in melanosome biogenesis and pathology
- PMID: 20381640
- PMCID: PMC2885761
- DOI: 10.1016/j.biocel.2010.03.023
Signaling pathways in melanosome biogenesis and pathology
Abstract
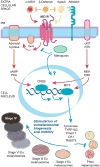
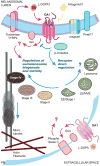
Melanosomes are the specialized intracellular organelles of pigment cells devoted to the synthesis, storage and transport of melanin pigments, which are responsible for most visible pigmentation in mammals and other vertebrates. As a direct consequence, any genetic mutation resulting in alteration of melanosomal function, either because affecting pigment cell survival, migration and differentiation, or because interfering with melanosome biogenesis, transport and transfer to keratinocytes, is immediately translated into color variations of skin, fur, hair or eyes. Thus, over 100 genes and proteins have been identified as pigmentary determinants in mammals, providing us with a deep understanding of this biological system, which functions by using mechanisms and processes that have parallels in other tissues and organs. In particular, many genes implicated in melanosome biogenesis have been characterized, so that melanosomes represent an incredible source of information and a model for organelles belonging to the secretory pathway. Furthermore, the function of melanosomes can be associated with common physiological phenotypes, such as variation of pigmentation among individuals, and with rare pathological conditions, such as albinism, characterized by severe visual defects. Among the most relevant mechanisms operating in melanosome biogenesis are the signal transduction pathways mediated by two peculiar G protein-coupled receptors: the melanocortin-1 receptor (MC1R), involved in the fair skin/red hair phenotype and skin cancer; and OA1 (GPR143), whose loss-of-function results in X-linked ocular albinism. This review will focus on the most recent novelties regarding the functioning of these two receptors, by highlighting emerging signaling mechanisms and general implications for cell biology and pathology.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
The ocular albinism type 1 protein, an intracellular G protein-coupled receptor, regulates melanosome transport in pigment cells.Hum Mol Genet. 2008 Nov 15;17(22):3487-501. doi: 10.1093/hmg/ddn241. Epub 2008 Aug 12. Hum Mol Genet. 2008. PMID: 18697795 Free PMC article.
-
Melanoregulin, product of the dsu locus, links the BLOC-pathway and OA1 in organelle biogenesis.PLoS One. 2012;7(9):e42446. doi: 10.1371/journal.pone.0042446. Epub 2012 Sep 11. PLoS One. 2012. PMID: 22984402 Free PMC article.
-
The ocular albinism type 1 (OA1) protein and the evidence for an intracellular signal transduction system involved in melanosome biogenesis.Pigment Cell Res. 2005 Aug;18(4):227-33. doi: 10.1111/j.1600-0749.2005.00240.x. Pigment Cell Res. 2005. PMID: 16029416 Review.
-
The ocular albinism type 1 (OA1) G-protein-coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition.Hum Mol Genet. 2009 Dec 1;18(23):4530-45. doi: 10.1093/hmg/ddp415. Epub 2009 Aug 28. Hum Mol Genet. 2009. PMID: 19717472
-
Ocular albinism type 1: more than meets the eye.Pigment Cell Res. 2001 Aug;14(4):243-8. doi: 10.1034/j.1600-0749.2001.140403.x. Pigment Cell Res. 2001. PMID: 11549106 Review.
Cited by
-
The Development of Sugar-Based Anti-Melanogenic Agents.Int J Mol Sci. 2016 Apr 16;17(4):583. doi: 10.3390/ijms17040583. Int J Mol Sci. 2016. PMID: 27092497 Free PMC article. Review.
-
Anti-Inflammation and Anti-Melanogenic Effects of Maca Root Extracts Fermented Using Lactobacillus Strains.Antioxidants (Basel). 2023 Mar 24;12(4):798. doi: 10.3390/antiox12040798. Antioxidants (Basel). 2023. PMID: 37107174 Free PMC article.
-
Comparison of Xiphophorus and human melanoma transcriptomes reveals conserved pathway interactions.Pigment Cell Melanoma Res. 2018 Jul;31(4):496-508. doi: 10.1111/pcmr.12686. Epub 2018 Jan 29. Pigment Cell Melanoma Res. 2018. PMID: 29316274 Free PMC article.
-
Up- or Downregulation of Melanin Synthesis Using Amino Acids, Peptides, and Their Analogs.Biomedicines. 2020 Sep 1;8(9):322. doi: 10.3390/biomedicines8090322. Biomedicines. 2020. PMID: 32882959 Free PMC article. Review.
-
Increased PHGDH expression promotes aberrant melanin accumulation.BMC Cancer. 2019 Jul 22;19(1):723. doi: 10.1186/s12885-019-5933-5. BMC Cancer. 2019. PMID: 31331318 Free PMC article.
References
-
- Abdel-Malek ZA. Agouti signal protein: crossing the ‘yellow’ signal and arriving to pathways that affect tumorgenesis. Pigment Cell Melanoma Res. 2009;22:253–4. - PubMed
-
- Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002;15:112–8. - PubMed
-
- Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP, Wakamatsu K, Thody AJ. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp Cell Res. 2001;268:26–35. - PubMed
-
- Aspengren S, Hedberg D, Wallin M. Melanophores: A model system for neuronal transport and exocytosis? J Neurosci Res. 2007;85:2591–600. - PubMed
-
- Barral DC, Seabra MC. The melanosome as a model to study organelle motility in mammals. Pigment Cell Res. 2004;17:111–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

