Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats
- PMID: 20382144
- PMCID: PMC2885460
- DOI: 10.1016/j.expneurol.2010.04.003
Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats
Abstract
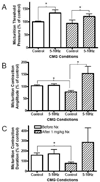
To determine the involvement of opioid receptors in the inhibitory pudendal-to-bladder reflex, the effect of naloxone (0.01-1 mg/kg, i.v.), an opioid receptor antagonist, on the inhibition of bladder activity evoked by pudendal nerve stimulation was investigated in alpha-chloralose anesthetized cats. The inhibition of reflex isovolumetric bladder contractions induced by pudendal nerve stimulation (5-10 Hz) at intensity threshold (T) for producing complete inhibition was significantly suppressed by naloxone at a high dose (0.3 mg/kg). However, the inhibition elicited at higher intensities (1.5-3 T) was not changed. Naloxone (1 mg/kg) did not alter the frequency dependence of the inhibitory effect of pudendal stimulation. During cystometrograms (CMGs) pudendal nerve stimulation significantly increased bladder capacity to 155.1+/-24.5% and 163.4+/-10% of the control at stimulation intensities of 1 T and 1.5-3 T, respectively. After administration of naloxone (1 mg/kg), the bladder capacity during pudendal nerve stimulation at inhibition threshold (1 T) was not significantly different from control, but it was significantly increased at higher intensities (1.5-3 T). Naloxone alone markedly reduced bladder capacity to 43+/-11.1% of the control, and pudendal stimulation completely reversed this facilitatory effect. This study revealed that activation of opioid receptors contributes to or facilitates the inhibitory pudendal-to-bladder reflex. The reduction in bladder capacity after naloxone treatment also indicates that endogenous opioid peptides mediate a tonic inhibition of micturition. Understanding the neurotransmitter mechanisms involved in the inhibitory pudendal-to-bladder reflex could promote the development of new treatments for bladder overactivity and incontinence.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures







Similar articles
-
Effect of methysergide on pudendal inhibition of micturition reflex in cats.Exp Neurol. 2013 Sep;247:250-8. doi: 10.1016/j.expneurol.2013.05.006. Epub 2013 May 18. Exp Neurol. 2013. PMID: 23688680 Free PMC article.
-
Propranolol, but not naloxone, enhances spinal reflex bladder activity and reduces pudendal inhibition in cats.Am J Physiol Regul Integr Comp Physiol. 2015 Jan 1;308(1):R42-9. doi: 10.1152/ajpregu.00368.2014. Epub 2014 Nov 12. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 25394827 Free PMC article.
-
Role of the brain stem in tibial inhibition of the micturition reflex in cats.Am J Physiol Renal Physiol. 2015 Aug 1;309(3):F242-50. doi: 10.1152/ajprenal.00135.2015. Epub 2015 May 27. Am J Physiol Renal Physiol. 2015. PMID: 26017973 Free PMC article.
-
Sacral neuromodulation blocks pudendal inhibition of reflex bladder activity in cats: insight into the efficacy of sacral neuromodulation in Fowler's syndrome.Am J Physiol Regul Integr Comp Physiol. 2018 Jan 1;314(1):R34-R42. doi: 10.1152/ajpregu.00285.2017. Epub 2017 Sep 20. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 28931549 Free PMC article.
-
Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats.Am J Physiol Renal Physiol. 2012 May 1;302(9):F1090-7. doi: 10.1152/ajprenal.00609.2011. Epub 2012 Jan 11. Am J Physiol Renal Physiol. 2012. PMID: 22237803 Free PMC article.
Cited by
-
Role of opioid and β-adrenergic receptors in bladder underactivity induced by prolonged pudendal nerve stimulation in cats.Neurourol Urodyn. 2023 Aug;42(6):1344-1351. doi: 10.1002/nau.25226. Epub 2023 Jun 12. Neurourol Urodyn. 2023. PMID: 37306331 Free PMC article.
-
Neural control of the lower urinary tract.Compr Physiol. 2015 Jan;5(1):327-96. doi: 10.1002/cphy.c130056. Compr Physiol. 2015. PMID: 25589273 Free PMC article. Review.
-
Spinal interneuronal mechanisms underlying pudendal and tibial neuromodulation of bladder function in cats.Exp Neurol. 2018 Oct;308:100-110. doi: 10.1016/j.expneurol.2018.06.015. Epub 2018 Jul 7. Exp Neurol. 2018. PMID: 30017972 Free PMC article.
-
A spinal GABAergic mechanism is necessary for bladder inhibition by pudendal afferent stimulation.Am J Physiol Renal Physiol. 2014 Oct 15;307(8):F921-30. doi: 10.1152/ajprenal.00330.2014. Epub 2014 Aug 20. Am J Physiol Renal Physiol. 2014. PMID: 25143456 Free PMC article.
-
Involvement of 5-HT3 receptors in pudendal inhibition of bladder overactivity in cats.Am J Physiol Renal Physiol. 2013 Sep 1;305(5):F663-71. doi: 10.1152/ajprenal.00105.2013. Epub 2013 Jul 3. Am J Physiol Renal Physiol. 2013. PMID: 23825079 Free PMC article.
References
-
- Booth AM, Hisamitsu T, Kawatani M, de Groat WC. Regulation of urinary bladder capacity by endogenous opioid peptides. J. Urol. 1985;133:339–342. - PubMed
-
- de Groat WC. The effect of glycine, GABA and strychnine on sacral parasympathetic preganglionic neurons. Brain Res. 1970;18:542–544. - PubMed
-
- de Groat WC. Inhibitory mechanisms in the sacral reflex pathways to the urinary bladder. In: Ryall RW, Kelly JS, editors. Iontophoresis and Transmitter Mechanisms in the Mammalian Central Nervous System. Holland: Elsevier; 1978. pp. 366–368.
-
- de Groat WC, Booth AM, Milne RJ, Roppolo JR. Parasympathetic preganglionic neurons in the sacral spinal cord. J. Auto. Nerv. Sys. 1982;5:23–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

