Role of MAP kinases in regulating expression of antioxidants and inflammatory mediators in mouse keratinocytes following exposure to the half mustard, 2-chloroethyl ethyl sulfide
- PMID: 20382172
- PMCID: PMC3582385
- DOI: 10.1016/j.taap.2010.04.001
Role of MAP kinases in regulating expression of antioxidants and inflammatory mediators in mouse keratinocytes following exposure to the half mustard, 2-chloroethyl ethyl sulfide
Abstract
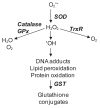
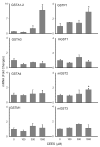
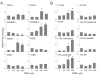
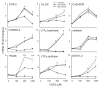
Dermal exposure to sulfur mustard causes inflammation and tissue injury. This is associated with changes in expression of antioxidants and eicosanoids which contribute to oxidative stress and toxicity. In the present studies we analyzed mechanisms regulating expression of these mediators using an in vitro skin construct model in which mouse keratinocytes were grown at an air-liquid interface and exposed directly to 2-chloroethyl ethyl sulfide (CEES), a model sulfur mustard vesicant. CEES (100-1000 microM) was found to cause marked increases in keratinocyte protein carbonyls, a marker of oxidative stress. This was correlated with increases in expression of Cu,Zn superoxide dismutase, catalase, thioredoxin reductase and the glutathione S-transferases, GSTA1-2, GSTP1 and mGST2. CEES also upregulated several enzymes important in the synthesis of prostaglandins and leukotrienes including cyclooxygenase-2 (COX-2), microsomal prostaglandin E synthase-2 (mPGES-2), prostaglandin D synthase (PGDS), 5-lipoxygenase (5-LOX), leukotriene A(4) (LTA(4)) hydrolase and leukotriene C(4) (LTC(4)) synthase. CEES readily activated keratinocyte JNK and p38 MAP kinases, signaling pathways which are known to regulate expression of antioxidants, as well as prostaglandin and leukotriene synthases. Inhibition of p38 MAP kinase suppressed CEES-induced expression of GSTA1-2, COX-2, mPGES-2, PGDS, 5-LOX, LTA(4) hydrolase and LTC(4) synthase, while JNK inhibition blocked PGDS and GSTP1. These data indicate that CEES modulates expression of antioxidants and enzymes producing inflammatory mediators by distinct mechanisms. Increases in antioxidants may be an adaptive process to limit tissue damage. Inhibiting the capacity of keratinocytes to generate eicosanoids may be important in limiting inflammation and protecting the skin from vesicant-induced oxidative stress and injury.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Efficacy of glutathione in ameliorating sulfur mustard analog-induced toxicity in cultured skin epidermal cells and in SKH-1 mouse skin in vivo.J Pharmacol Exp Ther. 2011 Feb;336(2):450-9. doi: 10.1124/jpet.110.173708. Epub 2010 Oct 25. J Pharmacol Exp Ther. 2011. PMID: 20974699 Free PMC article. Review.
-
Expression of proliferative and inflammatory markers in a full-thickness human skin equivalent following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide.Toxicol Appl Pharmacol. 2010 Dec 1;249(2):178-87. doi: 10.1016/j.taap.2010.09.005. Epub 2010 Sep 16. Toxicol Appl Pharmacol. 2010. PMID: 20840853 Free PMC article.
-
Sulfur mustard analog, 2-chloroethyl ethyl sulfide-induced skin injury involves DNA damage and induction of inflammatory mediators, in part via oxidative stress, in SKH-1 hairless mouse skin.Toxicol Lett. 2011 Sep 10;205(3):293-301. doi: 10.1016/j.toxlet.2011.06.019. Epub 2011 Jun 21. Toxicol Lett. 2011. PMID: 21722719 Free PMC article.
-
Relevance of Erk1/2-PI3K/Akt signaling pathway in CEES-induced oxidative stress regulates inflammation and apoptosis in keratinocytes.Cell Biol Toxicol. 2019 Dec;35(6):541-564. doi: 10.1007/s10565-019-09467-7. Epub 2019 Feb 25. Cell Biol Toxicol. 2019. PMID: 30805762
-
Changes in the oxidative stress/anti-oxidant system after exposure to sulfur mustard and antioxidant strategies in the therapy, a review.Toxicol Mech Methods. 2017 Jul;27(6):408-416. doi: 10.1080/15376516.2017.1320695. Epub 2017 May 5. Toxicol Mech Methods. 2017. PMID: 28413899 Review.
Cited by
-
Regulation of Hsp27 and Hsp70 expression in human and mouse skin construct models by caveolae following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide.Toxicol Appl Pharmacol. 2011 Jun 1;253(2):112-20. doi: 10.1016/j.taap.2011.03.015. Epub 2011 Mar 30. Toxicol Appl Pharmacol. 2011. PMID: 21457723 Free PMC article.
-
TrxR1 as a potent regulator of the Nrf2-Keap1 response system.Antioxid Redox Signal. 2015 Oct 1;23(10):823-53. doi: 10.1089/ars.2015.6378. Epub 2015 Jun 24. Antioxid Redox Signal. 2015. PMID: 26058897 Free PMC article. Review.
-
The Mixture of Salvianolic Acids from Salvia miltiorrhiza and Total Flavonoids from Anemarrhena asphodeloides Attenuate Sulfur Mustard-Induced Injury.Int J Mol Sci. 2015 Oct 15;16(10):24555-73. doi: 10.3390/ijms161024555. Int J Mol Sci. 2015. PMID: 26501264 Free PMC article.
-
Regulation of keratinocyte expression of stress proteins and antioxidants by the electrophilic nitrofatty acids 9- and 10-nitrooleic acid.Free Radic Biol Med. 2014 Feb;67:1-9. doi: 10.1016/j.freeradbiomed.2013.10.011. Epub 2013 Oct 15. Free Radic Biol Med. 2014. PMID: 24140437 Free PMC article.
-
Efficacy of glutathione in ameliorating sulfur mustard analog-induced toxicity in cultured skin epidermal cells and in SKH-1 mouse skin in vivo.J Pharmacol Exp Ther. 2011 Feb;336(2):450-9. doi: 10.1124/jpet.110.173708. Epub 2010 Oct 25. J Pharmacol Exp Ther. 2011. PMID: 20974699 Free PMC article. Review.
References
-
- Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. - PubMed
-
- Andreadis ST, Hamoen KE, Yarmush ML, Morgan JR. Keratinocyte growth factor induces hyperproliferation and delays differentiation in a skin equivalent model system. FASEB J. 2001;15:898–906. - PubMed
-
- Aufderheide M, Knebel JW, Ritter D. Novel approaches for studying pulmonary toxicity in vitro. Toxicol Lett. 2003;140-141:205–211. - PubMed
-
- Babin MC, Ricketts K, Skvorak JP, Gazaway M, Mitcheltree LW, Casillas RP. Systemic administration of candidate antivesicants to protect against topically applied sulfur mustard in the mouse ear vesicant model (MEVM) J Appl Toxicol. 2000;20(Suppl 1):S141–144. - PubMed
-
- Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA132624/CA/NCI NIH HHS/United States
- CA132624/CA/NCI NIH HHS/United States
- AR055073/AR/NIAMS NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- R01 CA100994/CA/NCI NIH HHS/United States
- CA093798/CA/NCI NIH HHS/United States
- R01 CA093798/CA/NCI NIH HHS/United States
- U54 AR055073/AR/NIAMS NIH HHS/United States
- ES005022/ES/NIEHS NIH HHS/United States
- GM034310/GM/NIGMS NIH HHS/United States
- R01 GM034310/GM/NIGMS NIH HHS/United States
- CA100994/CA/NCI NIH HHS/United States
- ES004738/ES/NIEHS NIH HHS/United States
- U54AR055073/AR/NIAMS NIH HHS/United States
- R01 ES004738/ES/NIEHS NIH HHS/United States
- R55 CA093798/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

