Microglial ablation and lipopolysaccharide preconditioning affects pilocarpine-induced seizures in mice
- PMID: 20382223
- PMCID: PMC2875881
- DOI: 10.1016/j.nbd.2010.04.001
Microglial ablation and lipopolysaccharide preconditioning affects pilocarpine-induced seizures in mice
Abstract
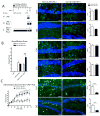
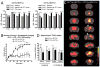
Activated microglia have been associated with neurodegeneration in patients and in animal models of Temporal Lobe Epilepsy (TLE), however their precise functions as neurotoxic or neuroprotective is a topic of significant investigation. To explore this, we examined the effects of pilocarpine-induced seizures in transgenic mice where microglia/macrophages were conditionally ablated. We found that unilateral ablation of microglia from the dorsal hippocampus did not alter acute seizure sensitivity. However, when this procedure was coupled with lipopolysaccharide (LPS) preconditioning (1 mg/kg given 24 h prior to acute seizure), we observed a significant pro-convulsant phenomenon. This effect was associated with lower metabolic activation in the ipsilateral hippocampus during acute seizures, and could be attributed to activity in the mossy fiber pathway. These findings reveal that preconditioning with LPS 24 h prior to seizure induction may have a protective effect which is abolished by unilateral hippocampal microglia/macrophage ablation.
(c) 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Akarsu ES, Ozdayi S, Algan E, Ulupinar F. The neuronal excitability time-dependently changes after lipopolysaccharide administration in mice: possible role of cyclooxygenase-2 induction. Epilepsy Res. 2006;71:181–7. - PubMed
-
- Altay EE, Fessler AJ, Gallagher M, Attarian HP, Dehdashti F, Vahle VJ, Ojemann J, Dowling JL, Gilliam FG. Correlation of severity of FDG-PET hypometabolism and interictal regional delta slowing in temporal lobe epilepsy. Epilepsia. 2005;46:573–6. - PubMed
-
- Arican N, Kaya M, Kalayci R, Uzun H, Ahishali B, Bilgic B, Elmas I, Kucuk M, Gurses C, Uzun M. Effects of lipopolysaccharide on blood-brain barrier permeability during pentylenetetrazole-induced epileptic seizures in rats. Life Sci. 2006;79:1–7. - PubMed
-
- Auvin S, Porta N, Nehlig A, Lecointe C, Vallee L, Bordet R. Inflammation in rat pups subjected to short hyperthermic seizures enhances brain long-term excitability. Epilepsy Res. 2009;86:124–30. - PubMed
-
- Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, Vezzani A. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol. 2005;57:804–12. - PubMed

