CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells
- PMID: 20382703
- PMCID: PMC2861083
- DOI: 10.2353/ajpath.2010.090759
CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells
Abstract
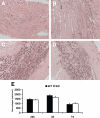
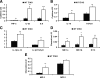
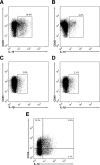
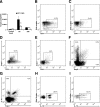
Myocardial infarction triggers an inflammatory reaction that is involved in cardiac remodeling. Cardiac repair is dependent on regulatory mechanisms that suppress inflammation and prevent excessive matrix degradation. Chemokine induction in the infarcted heart mediates recruitment of leukocyte subsets with distinct properties. We demonstrate that signaling through the CC chemokine receptor 5 (CCR5) prevents uncontrolled postinfarction inflammation and protects from adverse remodeling by recruiting suppressive mononuclear cells. CCR5 and its ligands macrophage inflammatory protein (MIP)-1alpha and MIP-1beta were markedly induced in the infarcted mouse myocardium. In addition, almost 40% of the mononuclear cells infiltrating the infarct expressed CCR5. CCR5(-/-) mice exhibited marked upregulation of proinflammatory cytokine and chemokine expression in the infarct. In wild-type infarcts CCR5+ mononuclear cells had anti-inflammatory properties, expressing higher levels of IL-10 than CCR5- cells. In contrast, mononuclear cells isolated from CCR5(-/-) infarcts had reduced IL-10 expression. Moreover, enhanced inflammation in the absence of CCR5 was associated with impaired recruitment of CD4+/foxp3+ regulatory T cells (Tregs). The CCR5+ Treg subset exhibited increased IL-10 expression, reflecting potent anti-inflammatory activity. Accentuated inflammation in CCR5(-/-) infarcts was associated with increased matrix metalloproteinase (MMP) expression, reduced TIMP levels, and enhanced MMP-2 and MMP-9 activity, resulting in worse cardiac dilation. These results suggest that CCR5-mediated Treg recruitment may restrain postinfarction inflammation, preventing excessive matrix degradation and attenuating adverse remodeling.
Figures



References
-
- Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. - PubMed
-
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. - PubMed
-
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

