TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells
- PMID: 20382709
- PMCID: PMC2877858
- DOI: 10.2353/ajpath.2010.090665
TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells
Abstract
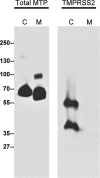
TMPRSS2, a type II transmembrane serine protease, is highly expressed by the epithelium of the human prostate gland. To explore the regulation and function of TMPRSS2 in the prostate, a panel of monoclonal antibodies with high sensitivity and specificity were generated. Immunodetection showed TMPRSS2 on the apical plasma membrane of the prostate luminal cells and demonstrated its release into semen as a component of prostasomes, organelle-like vesicles that may facilitate sperm function and enhance male reproduction. In prostate cancer cells, TMPRSS2 expression was increased and the protein mislocalized over the entire tumor cell membrane. In both LNCaP prostate cancer cells and human semen, TMPRSS2 protein was detected predominantly as inactive zymogen forms as part of an array of multiple noncovalent and disulfide-linked complexes, suggesting that TMPRSS2 activity may be regulated by unconventional mechanisms. Our data suggested that TMPRSS2, an apical surface serine protease, may have a normal role in male reproduction as a component of prostasomes. The aberrant cellular localization, and increased expression of the protease seen in cancer, may contribute to prostate tumorigenesis by providing access of the enzyme to nonphysiological substrates and binding-proteins.
Figures
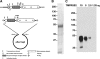

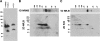



References
-
- Paoloni-Giacobino A, Chen H, Peitsch MC, Rossier C, Antonarakis SE. Cloning of the TMPRSS2 gene, which encodes a novel serine protease with transmembrane. LDLRA, and SRCR domains and maps to 21q223. Genomics. 1997;44:309–320. - PubMed
-
- Lin B, Ferguson C, White JT, Wang S, Vessella R, True LD, Hood L, Nelson PS. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59:4180–4184. - PubMed
-
- Jacquinet E, Rao NV, Rao GV, Zhengming W, Albertine KH, Hoidal JR. Cloning and characterization of the cDNA and gene for human epitheliasin. Eur J Biochem. 2001;268:2687–2699. - PubMed
-
- Hooper JD, Clements JA, Quigley JP, Antalis TM. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem. 2001;276:857–860. - PubMed
-
- Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, Antalis TM. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

