The evolutionary dynamics of operon distributions in eukaryote genomes
- PMID: 20382830
- PMCID: PMC2881147
- DOI: 10.1534/genetics.110.115766
The evolutionary dynamics of operon distributions in eukaryote genomes
Abstract
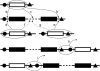
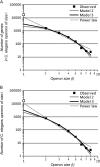
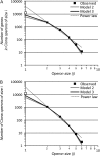
Genes in nematode and ascidian genomes frequently occur in operons--multiple genes sharing a common promoter to generate a polycistronic primary transcript--and such genes comprise 15-20% of the coding genome for Caenorhabditis elegans and Ciona intestinalis. Recent work in nematodes has demonstrated that the identity of genes within operons is highly conserved among species and that the unifying feature of genes within operons is that they are expressed in germline tissue. However, it is generally unknown what processes are responsible for generating the distribution of operon sizes across the genome, which are composed of up to eight genes per operon. Here we investigate several models for operon evolution to better understand their abundance, distribution of sizes, and evolutionary dynamics over time. We find that birth-death models of operon evolution reasonably describe the relative abundance of operons of different sizes in the C. elegans and Ciona genomes and generate predictions about the number of monocistronic, nonoperon genes that likely participate in the birth-death process. This theory, and applications to C. elegans and Ciona, motivates several new and testable hypotheses about eukaryote operon evolution.
Figures
References
-
- Blumenthal, T., 2004. Operons in eukaryotes. Brief. Funct. Genomic. Proteomic. 3 199–211. - PubMed
-
- Blumenthal, T., and K. S. Gleason, 2003. Caenorhabditis elegans operons: form and function. Nat. Rev. Genet. 4 112–120. - PubMed
-
- Blumenthal, T., D. Evans, C. D. Link, A. Guffanti, D. Lawson et al., 2002. A global analysis of Caenorhabditis elegans operons. Nature 417 851–854. - PubMed
-
- Charlesworth, B., and C. H. Langley, 1989. The population-genetics of Drosophila transposable elements. Annu. Rev. Genet. 23 251–287. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

